- Current Pediatric Research (2016) Volume 20, Issue 1
Severe Malaria due to Plasmodium vivax: Case Report
- *Corresponding Author:
- Vineeta Singh
National Institute of Malaria Research (ICMR), Sector-8, Dwarka, New Delhi, India.
E-mail: vineetas_2000@yahoo.com
| Date of Acceptance | April 05, 2016 |
Abstract
Background: Recently vivax severity has been on the rise in India where P. vivax contributes in equal ratio with P. falciparum to the malaria incidence. Objectives:We report here two cases of vivax malaria- one severe and another non-severe case, diagnosed and confirmed by microscopy, rapid diagnostic tests and 18S rRNA PCR assay.
Methods: Quantitative expression of two drug resistance genes (pvcrt-o, pvmdr1) and five vir (variant interspersed repeats) genes were measured simultaneously in these two cases for evaluating their role in disease pathogenesis. The non-severe case was taken as a control for measuring the expression levels of the studied genes in the severe case.
Results: The results indicated that clinical severe case was due to P.vivax only. The transcript levels of pvcrt-o and pvmdr1 alongwith the four vir genes were seen to be significantly high when compared to the non-severe vivax malaria case in the study.
Keywords
Severe vivax, Cerebral malaria, Drug resistance, vir genes.
Introduction
P. vivax is the most widespread species of human malaria across the world transmitted in 95 countries where an estimated 2.85 billion people are at risk of infection [1]. Although P. falciparum is responsible for majority of severe manifestations of malaria and mortality associated with it worldwide, P. vivax is no more considered benign as it is emerging to be equally complicated and lethal [2]. Many studies from India [3,4] as well as other parts of the world like Indonesian Papua [5,6], Papua New Guinea [7] and Brazilian Amazon [8,9] show a marked correlation between vivax infections, severe disease and death.
Previous studies have shown major clinical manifestations exclusively linked to severe P. vivax which include cerebral malaria, renal failure, circulatory collapse, ARDS, jaundice, severe anemia, thrombocytopenia, multiorgan dysfunction (MOD), possible coma which could clinically lead to life-threatening episodes [2,10]. Cerebral malaria and severe thrombocytopenia are reported as frequent clinical complications associated with vivax malaria, which were earlier exclusive for P. falciparum [11-13]. Parasitaemias in vivax infections are generally low and severe disease is not characterized by high parasite load [14]. Reports from different P. vivax endemic regions suggest the range and rate of severe clinical complications associated to P. vivax to be very diverse that could be due to many unexplained factors linked to the parasite, host and the type of infection [15].
Not much information is available on the genes that could have a role to play in the complicated vivax malaria. The vir (variant interspersed repeats) genes, the largest subtelomeric multigene superfamily found in P. vivax belonging to variant surface antigen (VSA) family, is proposed to have a role in the antigenic variation of the parasite and these genes are found to be highly variant [16,17]. Five vir genes were chosen for analysis in the present study according to their in silico data and a previous understanding of their speculated role in the pathogenesis of P. vivax [16,18,19].
Even though chloroquine resistance (CQR) in P. falciparum is found to be associated to mutations in pfcrt and pfmdr1 genes, the orthologous genes in P. vivax, P. vivax chloroquine resistance transporter (pvcrt-o) and the P. vivax multidrug resistance transporter (pvmdr1) are not yet considered as genetic markers for CQR in P. vivax [20,21]. However previous studies have shown significant increase in the expression levels of these genes in clinically severe vivax infections as well as chloroquine resistant cases suggesting a possible association of CQR and P. vivax severity [22,23]. The variant vir genes, pvcrt-o and pvmdr1 genes need to be explored as possible genetic markers for disease severity to gain a better understanding of the clinical and epidemiological mechanisms.
Here we present a case of a 14-year old girl suffering from severe vivax malaria presenting clinical complications and evaluate the expression of drug resistance genes (pvcrt-o, pvmdr1) and vir genes (vir 14-related, vir 12, vir 17-like, putative vir 14 and vir 10-related) normalized to a nonsevere vivax infection.
Case study
Patient 1
A 14-year old female was brought in and admitted to Kalawati Saran Children’s Hospital, a tertiary care hospital in New Delhi, in September 2013, with repeated convulsions since the past two days, giddiness, skin rash, muscle aches and recurring fever with chills. On arrival, the patient was febrile (38.3°C), confused and unresponsive. Family members revealed no prior history of convulsions. Vital signs included a blood pressure of 100/75 mm of Hg, 20 breaths per minute and a pulse rate of 110 beats per minute. Chest examinations were normal and spleen was palpable 3 cm below the costal margin. Laboratory investigations included the following results: hemoglobin = 6.8 g/dL (normal range:11.5-15.5 g/dL); platelets= 7100/μl (normal range:150,000-400,000 μl); total bilirubin = 2.9 mg/dL (normal range: 0.3- 1.9 mg/dL); serum creatinine = 1.2 mg/dL (normal range: 0.1- 1.0 mg/dL); total leukocyte count was 4900/μl with 30% neutrophils, 50% lymphocytes and 18% monocytes and serum electrolytes were within normal limits. Blood cultures and biochemical tests for other co-morbidities like serology against hepatitis A, hepatitis E, HIV, leptospirosis and dengue were performed and were found to be negative. Absence of other co-morbidities like pneumonia, enteric fever, varicella-zoster virus, diabetes, hypertension etc. was also confirmed. Lumbar puncture and CT scans were performed without any pathological findings.
Microscopic slides of thick and thin Giemsa-stained blood smears showed the presence of trophozoites and schizonts of P. vivax with 2.0% parasitaemia. Rapid diagnostic tests (RDTs) (FalciVax Zephyr Biomedical systems) also confirmed the presence of P. vivax in the blood sample. Two ml venous blood was collected from the patient and about 50 μl of the venous blood that was collected was used to make filter paper blood spots on Whatman filter paper (number 3) for the parasite genomic DNA extraction from QIAamp DNA Blood Mini Kit (Qiagen Inc.) according to the manufacturer’s instructions. A further diagnostic confirmation of P. vivax mono-infection was made by 18S rRNA nested PCR assay to amplify speciesspecfic sequences of the small subunit of rRNA genes of P.falciparum, P.vivax and P.malariae and also confirmed the absence of P. falciparum and P.malariae co-infection [24].
The tests confirmed that the patient was suffering from cerebral malaria and severe thrombocytopenia solely due to P. vivax. Patient was treated with injection ceftriaxone and artesunate in combination with primaquine along with intravenous (IV) fluids. Platelet transfusion was also administered. The patient made full recovery in six days.
Patient 2
A single uncomplicated vivax malaria case was used as control in this study who was treated in the same hospital and was discharged after all the tests were carried out. The uncomplicated P. vivax malaria patient was a 12-year old male who was brought in with recurring fever with chills (38°C) since the past three days and headache. Laboratory investigations showed all results within the normal range and preliminary diagnosis with RDT and microscopy revealed the presence of P. vivax infection. Thick and thin Geimsa-stained blood smears showed P. vivax asexual stages, trophozoites and schizonts with 1.5% parasitaemia. Diagnostic confirmation of Plasmodium species was done by 18S rRNA PCR assay which corroborated the results of microscopy and RDT showing P. vivax monoinfection in the patient. This uncomplicated vivax malaria patient was treated with chloroquine (25 mg/kg) for three days following which, the patient recovered.
The infected blood from both patients was passed through CF-11 column to remove the leukocytes. P. vivax total RNA was isolated by the QIAamp RNA Blood Mini Kit (Qiagen Inc.) according to the manufacturer’s instructions. First strand cDNA was then synthesized from 150 ng of total RNA using oligo (dT)18 primers (Thermo Scientific) according to the manufacturer’s protocol.
Relative quantification by real-time PCR was carried out to find the expression levels of five vir genes (vir 14-related, vir 12, vir 17-like, putative vir 14 and vir 10-related) and P. vivax drug resistance genes (pvcrt-o and pvmdr1). β-tubulin was used as the endogenous gene in this study as it has been used in similar studies previously [23]. Primers for pvcrt-o, pvmdr1, vir genes and β-tubulin were designed by Primer3web (v 4.0.0) software to compare the transcript levels of these genes [22]. The severe vivax isolate was normalized against the control uncomplicated vivax isolate by 2-ΔΔCt method for relative quantification of the drug resistance and vir genes.
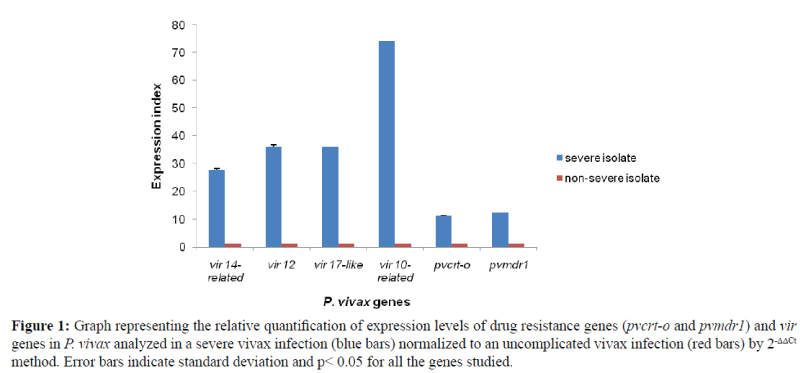
It was found that the expression levels of pvcrt-o, pvmdr1 and four out of five vir genes (vir 14-related, vir 12, vir 17-like and vir 10-related) were several fold higher in the severe vivax isolate as compared to the uncomplicated control isolate. The highest expression was seen in vir 10-related gene followed by vir 12, vir 17-like, vir 14-related, pvmdr1 and pvcrt-o genes (Figure 1). Putative vir 14 gene was not expressed in the test and control isolates.
Figure 1: Graph representing the relative quantification of expression levels of drug resistance genes (pvcrt-o and pvmdr1) and vir genes in P. vivax analyzed in a severe vivax infection (blue bars) normalized to an uncomplicated vivax infection (red bars) by 2-ΔΔCt method. Error bars indicate standard deviation and p< 0.05 for all the genes studied.
Discussion
The severe case of P. vivax reported here confirms the emerging severity due to vivax malaria in the country. Several reports from different endemic regions reveal diverse range and rate of occurrence of severe P. vivax clinical cases, which was earlier limited to P. falciparum infections only [2,5,6,8,9]. Cerebral malaria has been mainly associated with P. falciparum infections and very few such cases due to vivax have been reported only recently from several regions of the country and from other countries too [2,3,11,25,26].
Previous studies have elaborated that severe manifestations for malaria like cerebral malaria, hepatic dysfunction, acute renal failure and ARDS are caused by the sequestration of infected erythrocytes in deep vasculature of vital organs whereas thrombocytopenia and severe anemia are caused by factors like hemolysis, reduced cell deformability of infected and uninfected erythrocytes, increased splenic clearance, increased splenic uptake of platelets and decreased platelet production and survival [27,28]. Our patient exhibited cerebral malaria and severe thrombocytopenia exclusively due to P. vivax malaria as stated previously in the manuscript. Previous data indicates that P. vivax can cause both sequestration and non-sequestration related clinical manifestations [2]. In several studies, severe anemia has been the most frequently associated manifestation in severe P. vivax [2,6,8,13]. Also, it has been observed that the occurrence of thrombocytopenia in P. vivax malaria is on the rise [12,29,30]. During the preliminary examination of a febrile patient, if thrombocytopenia is also observed it should be borne in mind to get the patient investigated for malaria infection. The occurrence of two or more severity criteria is now frequently observed in severe vivax cases [31]. Similarly in our severe case, we observed two severe clinical manifestations i.e. convulsions and thrombocytopenia which have also been seen elsewhere [2,11-13,25,26]. It was not possible in our study to estimate the total parasite biomass which was a limitation in our study though, in the severe malaria criteria bilirubin and low hemoglobin levels are important diagnostic markers and should not be excluded in the initial diagnosis of P. vivax infected patients.
The expression levels of the drug resistance genes viz. pvcrt-o and pvmdr1 possibly having a role in chloroquine resistance and disease severity was seen to be significantly high when compared to the non-severe vivax malaria case in the study. The pvcrt-o gene expression was 11.16 fold higher and pvmdr1 gene expression was 12.37 fold higher in severe vivax case normalized to the non-severe infection.
Species specific 18S rRNA PCR technique confirmed that P. vivax alone was responsible for the severe manifestations of the patient under study. Many studies have been conducted in the past from Papua Indonesia, Manaus Brazil, Venezuela and India that have highlighted the occurrence of severe P. vivax infections leading to life threatening episodes more in children as compared to adults in regions of high vivax transmission [6,9,32,33]. Lanca et al., observed severe anemia and respiratory distress to be the most frequent complications in their study.
We require more comprehensive studies to elucidate the role of drug resistance and virulence genes in severe vivax infections. The molecular mechanisms need to be unraveled for detailed understanding of the disease pathogenesis of vivax malaria, which is now severe like P. falciparum. The malaria burden due to P. vivax needs to be examined urgently for implementation of adequate control measures in the national control program.
Acknowledgements
This work was supported by the Indian Council of Medical Research [F/801/2010-ECD-II, ICMR/5/8-7(247)/V- 2012-ECD-II]. We would also like to acknowledge the patients included in the study.
References
- Gething PW, Elyazar IR, Moyes CL, et al. A long neglected world malaria map: Plasmodium vivaxendemicity in 2010. PLoSNegl Trop Dis 2012; 6: e1814.
- Kochar DK, Das A, Kochar SK, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 2009; 80: 194-198.
- Kochar DK, Tanwar GS, Khatri PC, et al. Clinical features of children hospitalized with malaria- a study from Bikaner, northwest India. Am J Trop Med Hyg 2010; 83: 981-989.
- Sharma R, Gohain S, Chandra J, et al. Plasmodium vivax malaria admissions and risk of mortality in a tertiary-care children’s hospital in North India. PaediatrInt Child Health 2012; 32: 152-157.
- Barcus MJ, Basri H, Picarima H, et al. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg 2007; 77: 984-991.
- Tjitra E, Anstey NM, Sugiarto P, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 2008; 5: e128.
- Genton B, D'Acremont V, Rare L, et al. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 2008; 5: e127.
- Alexandre MA, Ferreira CO, SiqueiraAM,et al. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis 2010; 16: 1611-1614.
- Lanca EF, Magalhaes BM, Vitor-Silva S, et al. Risk factors and characterization of Plasmodium vivax-associated admissions to pediatric intensive care units in the Brazilian Amazon. PLoS One 2012; 7: e35406.
- Lacerda MV, Fragoso SC, Alecrim MG, et al. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis 2012; 55: e67-74.
- Beg MA, Khan R, Baig SM, et al. Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg 2002; 67: 230-232.
- Muley A, Lakhani J, Bhirud S, et al. Thrombocytopenia in Plasmodium vivax malaria: How significant? J Trop Med 2014; 2014: 567469.
- Rodriguez-Morales AJ, Sanchez E, Vargas M, et al. Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J Trop Pediatr 2006; 52: 49-51.
- Anstey NM, Russell B, Yeo TW, et al. The pathophysiology of vivax malaria. Trends Parasitol 2009; 25: 220-227.
- Baird JK. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. ClinMicrobiol Rev 2013; 26: 36-57.
- Gupta P, Das A, Singh OP, et al. Assessing the genetic diversity of the vir genes in Indian Plasmodium vivax population. Acta Trop 2012; 124: 133-139.
- Gupta P, Pande V, Das A, et al. Genetic polymorphisms in VIR genes among Indian Plasmodium vivax populations. Korean J Parasitol 2014; 52: 557-564.
- Bernabeu M, Lopez FJ, Ferrer M, et al. Functional analysis of Plasmodium vivax VIR proteins reveals different subcellular localizations and cytoadherence to the ICAM-1 endothelial receptor. Cell Microbiol 2012; 14: 386-400.
- Neafsey DE, Galinsky K, Jiang RH, et al. The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet 2012; 44: 1046-1050.
- Fidock DA, Nomura T, Talley AK, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell 2000; 6: 861–871.
- Suwanarusk R, Russell B, Chavchich M, et al. Chloroquine resistant Plasmodium vivax: in vitro characterization and association with molecular polymorphisms. PLoS One 2007; 2: e1089.
- Fernandez-Becerra C, Pinazo MJ, Gonzalez A, et al. Increased expression levels of the pvcrt-o and pvmdr1 genes in a patient with severe Plasmodium vivax malaria. Malar J 2009; 8: 55.
- Melo GC, Monteiro WM, Siqueira AM, et al. Expression levels of pvcrt-o and Pvmdr-1 are associated with chloroquine resistance and severe Plasmodium vivax malaria in patients of the Brazilian Amazon. PLoS One 2014; 9: e105922.
- Snounou G, Viriyakosol S, Zhu XP, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. MolBiochemParasitol 1993; 61: 315-320.
- Vinod KV, Talari K, Gopalakrishnan M, et al. Unusual presentations of vivax malaria: A report of two cases. J Vector Borne Dis 2012; 49: 49-51.
- Anvikar AR, Singh DK, Singh R, et al. Vivax malaria presenting with cerebral malaria and convulsions. ActaParasitol 2010; 55: 96-98.
- Kochar DK, Saxena V, Singh N, et al. Plasmodium vivax malaria. Emerg Infect Dis 2005; 11: 132-134.
- Narang GS, Singla N. Thrombocytopenia and other complications of Plasmodium vivax malaria. CurrPediatr Res 2011; 15: 117-119.
- Coelho HC, Lopes SC, et al. Thrombocytopenia in Plasmodium vivax malaria is related to platelets phagocytosis. PLoS One 2013; 8: e63410.
- Kochar DK, Das A, Kochar A, et al. Thrombocytopenia in Plasmodium falciparum, Plasmodium vivax and mixed infection malaria: a study from Bikaner (Northwestern India). Platelets 2010; 21: 623-627.
- Siqueira AM, Lacerda MV, Magalhaes BM, et al. Characterization of Plasmodium vivax-associated admissions to reference hospitals in Brazil and India. BMC Med 2015; 13: 57.
- Rodriguez-Morales AJ, Benitez JA, Arria M. Malaria mortality in Venezuela: focus on deaths due to Plasmodium vivax in children. J Trop Pediatr 2008; 54: 94-101.
- Kochar DK, Tanwar GS, Khatri PC, et al. Clinical features of children hospitalized with malaria- a study from Bikaner, northwest India. Am J Trop Med Hyg 2010; 83: 981-989.