Research Article - Biomedical Research (2017) Volume 28, Issue 17
Serum prolidase activity as an indicator of liver fibrosis in chronic hepatitis B infection
Suleyman Kaleli1*, Oguz Karabay2, Ertugrul Guclu2 and Meltem Karabay3
1Department of Medical Biology, Faculty of Medicine, Sakarya University, Sakarya, Turkey
2Department of Infectious Diseases and Clinical Microbiology, Faculty of Medicine, Institute of Medical Sciences, Sakarya University, Sakarya, Turkey
3Department of Paediatrics and Neonatology, Faculty of Medicine, Sakarya University, Sakarya, Turkey
- *Corresponding Author:
- Suleyman Kaleli
Department of Medical Biology
Faculty of Medicine
Sakarya University
Sakarya, Turkey
Accepted on August 16, 2017
Abstract
Background/Aims: Hepatitis B Virus (HBV) infections cause chronic infection, cirrhosis, and hepatocellular carcinoma. In this study, we aimed to demonstrate that the Serum Prolidase Activity (SPA) is one of the indicators of chronic hepatitis B related liver fibrosis.
Materials and Methods: Diagnosis of prolonged hepatitis B (CHB) was determined with hepatitis B surface antigen (HBsAg) positivity for more than 6 months. All CHB cases were followed up with liver enzymes (ALT, SAT, GGT, bilirubin), HBV DNA and sonography at least once a year. Patients, who were pregnant, or taking sclerosing treatment or cancer treatment and treatment of diabetes were excluded from the study. The control group was designated randomly from individuals’ not caring hepatitis B surface antigen. SPSS 20 was used for statistical analysis. A p value was <0.05 was used meaningful.
Results: A total 30 patients CHB (the average age 38.53 ± 8.99; 19 female/11 male) and 30 healthy controls (the average age 35.80 ± 9.31; 14 female/16 male) were included in the study. While ALT (38.1 versus 23.3 U/L), ALP (71.4 versus 48.7 U/L), PLT (213.2 versus 269.2 unit/ml) and SPA levels (1263.8 versus 784.6 U/L) were found expressively diverse in the study group than in control group (p=0.049, p=0.0001, p=0,037 and p=0.0001, respectively).
Conclusions: SPA activity is considerably increased in CHB patients having liver damage. So, we think that SPA can be candidate as a non-invasive biomarker for liver fibrosis.
Keywords
Serum prolidase activity, Fibrosis, Liver damage, Hepatitis B.
Introduction
Hepatitis B Virus (HBV) infection is one of the serious health concerns around the world. Approximately two billion people infected with HBV in the worldwide. Chronic HBV infection (CHB) is closely related with progression of hepatocellular carcinoma and cirrhosis [1-3].
It is showed that Liver’s Stellate Cells (LSC) is a main factor as a fibrogenic response [4]. These cells contain a small number of oil droplets, Granular Endoplasmic Reticulum (GER) with enlarged volume and the Golgi apparatus. These cells are also called as fat-storing cells, lipocytes and perisinusoidal cell. Developing inflammation during viral hepatitis illness is important trigger for fibrogenic response and cirrhosis. The expansion of cirrhotic LSC groups in the liver occurs with myogenic fibroblastic differentiation [5]. It is known that liver fibrosis causes portal hypertension, impaired synthetic function, and ultimately leads to decreased cell life. Fibrogenic response can be reversed. Clear improvement in liver histology was demonstrated resulting long-term antiviral treatment in long-lasting hepatitis B and C [6].
Prolidase is a manganese dependent metalloproteinase enzyme that located in many tissues such as intestinal mucosa, kidney, liver, brain, heart, uterus, thymus, erythrocytes, leukocytes, fibroblasts and plasma. Prolidase enzyme (Enzyme code, 3.4.13.9) is responsible for the destruction of dipeptides containing C-terminal proline and hydroxyproline. These peptides related with in the last stage of protein catabolism. Chronic repeated infection, mental deficiency, splenomegaly and dermatologic lesions can be seen in the absence of prolidase activity. Proline and hydroxyproline amino acid groups which are liberated by the prolidase enzyme constitute about 25% of the collagen tissue. Prolidase is also necessary for the maintenance of connective tissue [7-10]. So, in the presence of the liver fibrosis, it is expected to increase in prolidase enzyme activity. In this study, we tried to demonstrate that the Serum Prolidase Activity (SPA) is one of the indicators of chronic hepatitis B related liver fibrosis.
Materials and Methods
The study was performed at the Medicine Faculty, Sakarya University, Turkey. The subjects were provided by the Department of Infectious Diseases Clinic. Our institutional ethical committee approved this study (Number: 71522473/050.01.04/143, Date: 03/08/2016) and informed consent was procured from all participating subjects in the study.
Study center and cases
This study was performed on 30 patients with chronic HBV (study group) and 30 healthy control (control group) cases who are applicant of Infectious Diseases Clinic in Sakarya University Hospital. Diagnosis of chronic hepatitis B was made by HBsAg positivity in the blood for more than 6 months. Study group was followed up with liver enzymes, HBV DNA and ultrasonography at least once a year.
Inclusion criterions:
1. Between 18-70 y
2. Non-pregnant
3. Non-taking sclerosing treatment
4. Non-cancer history
5. Non-diabetics
Sample collection
Morning blood samples taken from the arm vein were transferred into our laboratory and kept at -80°C until the day of analysis.
Analysis of platelet
Platelets (PLT) in which blood samples collected in a hematologic sample tube containing anticoagulant were investigated by using the Cell-Dyn 3700 SL haematology analyzer (Abbott Laboratories, North Chicago, IL, USA). Normal PLT count was accepted between 150.000-400.000 K/uL.
Analysis of biochemical parameters
Serum samples kept at -80°C were removed from deepfreeze and dissolved at room temperature. The serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT) and total bilirubin analysis were performed colorimetrically at Abbott Architect laboratory system with using Architect brand commercial kits. Normal ALT and AST values were accepted as <50 U/L.
HBV DNA test
HBV DNA test was studied with using real-time reverse transcriptase PCR (TCycler-PCR; BioRad, USA).
Histopathology activity index (HAI)
Histopathology activity index (HAI) and fibrosis score were determined as defined by Knodell et al. [11]. ALT levels at the time of liver biopsy were recorded.
Prolidase activity
Optimized method of Myara et al. was used [12,13]. Briefly 500 μL pre-incubation solution containing 1 mmol/L GSH, 5 mmol/L MnCl2, 0.1% Triton-X100 and 50 mmol/L Tris-HCl buffer (pH 7.8) was incubated with 100 μL serum for 3 h at 37°C. 100 μL of the mixture was taken and 100 μL pre-incubation solution containing 144 mmol/L Gly-Pro was added, then they were incubated for 30 min at 37°C. The reaction was ceased by adding 1 ml of 0.45 mol/L TCA into the mixture. The mixture was whizzed at 1500xg for 10 min to separate supernatants. 1 ml of modified chinard solution was added onto 0.5 ml of the supernatant. It was mixed in vortex for 30 s. The mixture was incubated at 90°C for 20 min. Then the absorbance of mixture was measured immediately at 515 nm in a spectrophotometer (Beckman Coulter DU 530 UV/Vis Spectrophotometer, USA) against the blank solution. The measured proline concentration was calculated comparing with 5 mg/dl L-proline used as a standard.
Statistical analysis
HAI scores were compared by Mann-Whitney rank sum test and Fisher’s exact test and ALT values by Mann-Whitney rank sum test [14]. Kolmogorov-Smirnov test was used to denote whether a normal distribution of variables. T test for independent sample groups was used. Mann-Whitney U test for nonparametric continuous data was used. The continuous data were presented as the mean ± standard deviation. A p-value< 0.05 was considered significant. Analyses were accomplished by using commercial software (IBM SPSS Statistics, Version 22.0. Armonk, NY: IBM Corp.)
Results
The average age of study group was 38.53 ± 8.99 y (19 females/11 males) while it was 35.80 ± 9.31 y (14 females/16 males) in the control group.
AST, ALT, ALP, GGT, total bilirubin, PLT and serum prolidase activity levels of total 60 subjects were shown in Table 1.
| Parameter | Study group N=30 (mean ± standard deviation) | Control group N=30 (mean ± standard deviation) | P |
|---|---|---|---|
| Sex F/M | 43058 | 14/16 | - |
| Average of age | 38.53 ± 8.99 | 35.80 ± 9.31 | 0.05 |
| Aspartate aminotransferase (U/L) | 27.53 ± 13.40 | 26.03 ± 12.12 | 0.05 |
| Alanine aminotransferase (U/L) | 38.10 ± 38.68 | 23.27 ± 11.75 | 0.049 |
| Alkaline phosphatase (U/L) | 71.40 ± 19.80 | 48.73 ± 25.98 | 0.0001 |
| Gamma-glutamyl transferase (U/L) | 27.57 ± 20.07 | 30.20 ± 19.18 | 0.05 |
| Total bilirubin (mg/dl) | 0.67 ± 0.28 | 0.51 ± 0.47>0.05 | 0.05 |
| Platelet (K/uL) | 213.20 ± 68.13 | 269.20 ± 73.52 | 0.037 |
| Serum prolidase activity (U/L) | 1263.80 ± 151.26 | 784.60 ± 144.88 | 0.0001 |
| HBVDNA (Log10/IU) | 4.084 ± 2.14 | - | - |
Table 1. Demographic and laboratory properties of the study subjects.
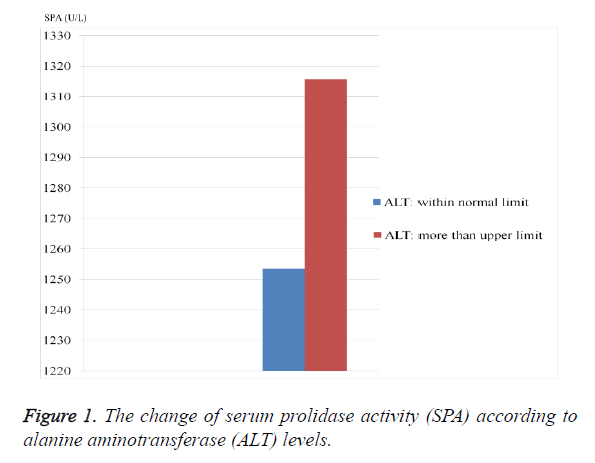
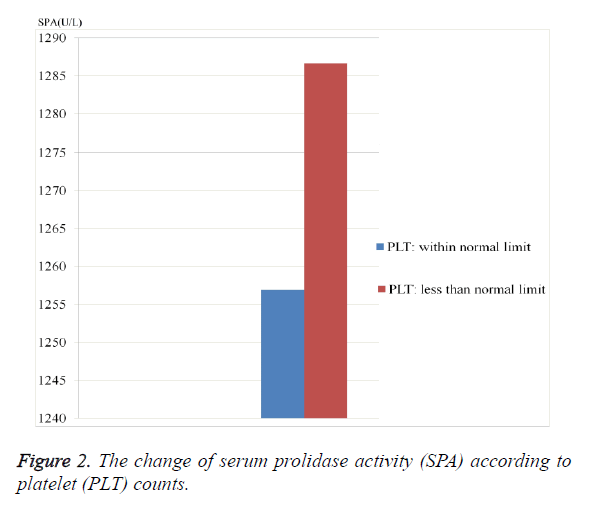
As seen from the Table 1, ALT, ALP and SPA levels were considerably higher in HBV-group than control group (p=0.049, 0.0001 and 0.0001; respectively). The PLT value was significantly lower in HBV-group (p=0.037). SPA level was significantly higher in patients who have higher ALT level and in patients who have lower PLT level (Figures 1 and 2).
Discussion
Accumulation of mainly interstitial collagen and other matrix components play a role on liver fibrosis. Normally the production and degradation of extracellular matrix stay in balance. However, it is broken during the fibrosis process [15]. Therefore, we think that the increase in necessity of SPA and collagen is correlated with the increased fibrosis in liver. Our results confirmed our theory and we found that there is a clear relationship between the SPA and liver destruction indicators. Due to provide basis for progression of cirrhosis or hepatocellular carcinoma, diagnosis of HBV infectious is crucial point for treatment of liver diseases. At some studies, it was demonstrated that the diagnosis of HBV infectious depends on presence of HBV DNA, the level of total bilirubin and the level of liver enzymes as ALT, AST, GGT and ALP [16-22]. In the case of increasing liver cell damage, the level of liver enzymes has been increasing, at the same time chronic inflammation has been increasing. In this study, it was found that the level of SPA is high in the subjects with hepatitis B having thrombocytopenia, high level of ALT and ALP.
With growing liver inflammation, the collagen degradation also accrues dramatically. So, SPA level is increasing with inflammation as seen at our study. This is because prolidase is an extracellular matrix element and cytosolic enzyme that is responsible for destruction of intra-extracellular collagen [23]. The level of SPA is significantly higher in the cases with chronic liver disease and clarified liver cell destruction. It is suggested that augmented collagen catalysis in the damaged liver cells leads to an increase in SPA level.
In a study, Sen et al. discovered that the SPA levels were significantly higher in children with chronic hepatitis compared to the controls [24]. Similar with us, the researchers found a significant correlation between ALT and SPA. Also, the low PLT value was related with high SPA level.
Duygu et al. studied SPA levels in chronic Hepatitis B infected patients and they demonstrated that there is an increase in SPA with chronic hepatitis infection [25]. Nazligul et al. compared the liver biopsy with SPA level in patients with chronic viral hepatitis and found out that SPA level is associated with genesis of fibrosis and is more useful technique for diagnosis of the patients with chronic viral hepatitis than liver biopsy [26].
In cirrhotic patients, the relationship between the severity of cirrhosis and the progress of thrombocytopenia has been shown previous studies [5]. Also, we observed that activity of SPA was higher in patients who had advanced chronic liver disease with thrombocytopenia. According to our results, it is suggested that the detection of high level of SPA in patients having thrombocytopenia may be an important biomarker in showing forwardness of disease.
Consequently, it is thought that SPA activity significantly rises in liver damage related with chronic HBV and the level of SPA may be used as a non-invasive biomarker for estimation of fibrosis in the liver. At the same time, we also think that SPA can be used as an indicator of inflammation of the liver in HBV positive patients.
Conflict of Interest
The authors declare no conflict of interest.
References
- Della Corte C, Nobili V, Comparcola Cainelli DF, Vento S. Management of chronic hepatitis B in children: an unresolved issue. J Gastroenterol Hepatol 2014; 29: 912-919.
- Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol 2010; 58: 258-266.
- In Who Member States. Global policy report on the prevention and control of viral hepatitis. World Health Organization 2013.
- Li F, Zhu CL, Zhanq H. Role of hyaluronic acid and laminin as serum markers for predicting significant fibrosis in patients with chronic hepatitis B. Brazilian J Infect Dis 2012; 16: 9-14.
- Enzan H, Himeno H, Iwamura S. Immunohistochemical identification of Ito cells and their myofibroblastic cellular matrix and proliferation of transformation in adult human liver. Virchows Archiv 1994; 424: 249-256.
- Poynard T, McHutchison J, Manns M. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterol 2002; 122: 1303-1313.
- Endo FA, Matsuda I. Molecular basis of prolidase (peptidase D) deficiency. Mol Biol Med 1991; 8: 117-127.
- Myara I, Cosson C, Moatti Lemonnier NA. Human kidney prolidase-purification, pre-incubation properties and immunological reactivity. Int J Biochem 1994; 26: 207-214.
- Whang SH, Zhi QW, Sun MJ. Dual activities of human prolidase. Toxicol In Vitro 2006; 20: 71-77.
- Davis NC, Smith EL. Purification and some properties of prolidase of swine kidney. J Biol Chem 1957; 224: 261-275.
- Knodell GR, Ishak GK, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatol 1981; 1: 431-435.
- Ozcan O, Gultepe M, İpcioglu OM, Bolat B, Kayadibi H. Optimization of the photometric enzyme activity assay for evaluating real activity of prolidase. Turkish J Biochem 2007; 32: 12-16.
- Myara I, Charpentier C, Lemonnier A. Optimal conditions for prolidase assay by proline colorimetric determination: Application to iminodipeptiduria. Clinica Chimica Acta 1982; 125: 193-205.
- Lindh M, Hannoun C, Dhillon AP, Norkrans G, Horal P. Core promoter mutations and genotypes in relation to viral replication and liver damage in East Asian Hepatitis B virus carriers. J Infect Dis 1999; 179: 775-782.
- Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 2004; 1: 98-105.
- Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, Isaguliants MG. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget 2017; 8: 3895-3932.
- Rotman Y, Brown TA, Hoofnagle JH. Evaluation of the patient with hepatitis B. Hepatol 2009; 49: 22-27.
- Uluca Ü, Şen V, Ece A, Tan İ, Karabel D, Aktar F, Karabel M, Balık H, Güneş, A. Serum galectin-3 levels in children with chronic hepatitis B infection and inactive hepatitis B carriers. Med Sci Monitor 2015; 21: 1376-1380.
- Qian L, Wang W, Zhou Y, Ma J. Effects of reduced glutathione therapy on chronic hepatitis B. Central-Eur J Immunol 2017; 42: 97-100.
- Williams ALB, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis relationship to cirrhosis. Gastroenterol 1988; 95: 734-739.
- Torres-Valadez R, Roman S, Jose-Abrego A, Sepulveda-Villegas M, Ojeda-Granados C, Rivera-Iñiguez I, Panduro A. Early detection of liver damage in Mexican patients with chronic liver disease. J Transl Intern Med 2017; 5: 49-57.
- Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, Weiss M, Wolfgang CL, Choti MA, Pawlik TM. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol 2014; 21: 147-154.
- Kurien BT, Patel NC, Porter AC, D’Souza A, Miller D, Matsumoto H, Wang H, Scofield RH. Prolidase deficiency and the biochemical assays used in its diagnosis. Anal Biochem 2005; 349: 165-175.
- Sen V, Uluca U, Ece A, Kaplan I, Bozkurt F, Aktar F, Bag S, Tekin R. Serum prolidase activity and oxidant-antioxidant status in children with chronic hepatitis B virus infection. Italian J Pediatr 2014; 40: 2-8.
- Duygu F, Aksoy N, Cicek AC, Butun I, Unlu S. Does prolidase indicate worsening of hepatitis B infection? J Clinl Laboratory Analysis 2013; 27: 398-401.
- Nazligul Y, Aslan M, Taskın A, Aksoy N. Serum prolidase activity may be an index of liver fibrosis in chronic viral hepatitis. Eastern J Med 2015; 20: 125- 130.