- Biomedical Research (2009) Volume 20, Issue 2
Serum prolactin levels in women with rheumatoid arthritis
Sucheta Ghule*, Archana Dhotre, Madhur Gupta, Prashant Dharme, S.M. VaidyaDepartment of Biochemistry, Government Medical College, Nagpur, India.
- *Corresponding Author:
- Sucheta Ghule
Lecturer in Biochemistry
Govt. Medical College Nagpur
Maharashtra, India
Accepted March 01 2009
Abstract
Numerous studies state the role of prolactin, a hormone of anterior pituitary in autoimmune response of rheumatoid arthritis. Postpartum exacerbation of arthritis suggests the relation of prolactin with autoimmune arthritis. The present study was carried out to see the status of serum prolactin in patients of rheumatoid arthritis attending Shree Ayurvedic College and Government Medical College, Nagpur. A case control study was carried out with 20 women of rheumatoid arthritis (Group A) and. 20 apparently healthy age- sex- matched volunteers (Group B).The diagnosis was confirmed by revised ACR criteria for rheumatoid arthritis. Fasting blood samples (5 ml/subject) were collected from the cases and controls. The serum was separated and stored in deep freeze at – 20°C Along with other parameters serum prolactin concentrations were measured by Sandwich Enzyme immuno-assay system (ELISA). Statistical evaluation was done by student’s t test and p-value was calculated.(p < 0.001). The serum prolactin levels in cases were significantly increased than in controls. Many studies indicate the role of prolactin in the autoimmune responses. Although exact mechanism is not known, it is proposed that prolactin is secreted by synovial cells and lymphocytes which do have dopamine receptors. Also remission of arthritis with anti- prolactin drugs suggests the role of prolactin in rheumatoid arthritis. In conclusion, elevated serum prolactin levels in the cases of rheumatoid arthritis may suggest its possible role in autoimmune response
Keywords
Prolactin, autoimmune, rheumatoid arthritis
Introduction
Prolactin is a hormone of the anterior pituitary gland which induces lactation in response to the suckling stimulus of young ones.
Synthesis and secretion of prolactin is not restricted to the anterior pituitary gland, but other organs and tissues in the body have this capability. Prolactin has been found to be secreted by the immune system, the central nervous system, the uterus and the mammary gland itself [1]. Biochemically, prolactin is found in different sizes and in different post- translational forms such as phosphorylated or glycosylated forms. The versatile roles and sources of prolactin had led Bern and Nicoll [2] to suggest renaming it “versatilin and omnipoitin”. Apart from reproduction it has been shown to control a variety of behaviors and even play a role in homeostasis.
The role of prolactin in the immune response was demonstrated for the first time in 1972. It proved that exogenous prolactin enhanced thymic function in prolactin-deficient dwarf mice [3].
Shortly thereafter, Nagy and Berczi [4] found that hypophysectomy or suppression of prolactin secretion with bromocryptine led to decrease of humoral or cell-mediated immunity which was reversed by treatment with prolactin.
Researchers are focused on the role of prolactin in the immune response. They are based on the fact that prolactin enhanced immune responses in vivo. Rheumatoid arthritis is already known to be an autoimmune disease. It has been suggested that excessive prolactin secretion may contribute to the pathogenesis of rheumatoid arthritis [5,6,7]. During gestation there is remission of rheumatoid arthritis which is exacerbated in the postpartum period. During first trimester the prolactin Prolactin in arthritis concentrations are low which start rising from second trimester of gestation. They reach their peak at the end of pregnancy, This increased levels of prolactin have been related to the post-partum exacerbation of rheumatoid arthritis, suggesting the role of prolactin in autoimmune arthritis [8].
We have therefore carried out the following study to estimate serum prolactin levels in patients of rheumatoid arthritis and compare them with the normal healthy controls.
Material and Methods
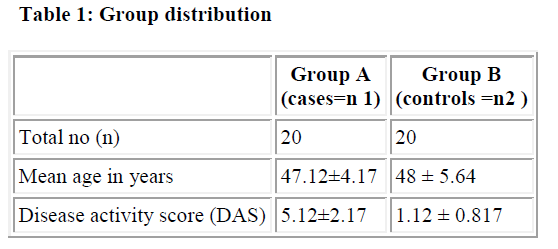
We included 20 female patients with rheumatoid arthritis attending Govt. Medical Hospital and 20 age-matched healthy female controls with age group between 45 to 60 years. All participants voluntarily participated in the study with informed consent approved by the ethical committee of the institution. All the cases were screened for rheumatoid factor, C-reactive proteins, ESR, X-ray of the joint involved. The patients fulfilled the revised ACR (American college of Rheumatology) criteria for rheumatoid arthritis [9].
In patients with rheumatoid arthritis, disease activity was assessed using a disease activity score (DAS) which included the erythrocyte sedimentation rate (ESR) and the number of swollen joints, tender joints and general health. Using this data, the DAS can be calculated using the following formula: DAS (DAS = 0.56٭ sqrt (tender) + 0.28٭ sqrt (swollen) + 0.70٭ ln (ESR) + 0.014٭ GH). The DAS provides a number on a scale from 0 to 10 indicating the current activity of the rheumatoid arthritis of the patient. Cases with DAS above 3.2 were included in the study [10,11]. Patients treated with a Disease Modifying Anti- Rheumatic Drugs (DMARD) or with oral, intramuscular, intra-articular glucocorticoids, or oral contraceptives and NSAIDs at least two week before the study were excluded from the study. Also pregnant and postpartum patients were excluded from the study.
Serum prolactin concentrations were measured under basal conditions and all blood samples were collected in dry tubes. Blood samples were centrifuged at 3000 rpm for 5 minutes and serum was stored in deep freeze at -20 °C until analysis. Serum prolactin concentrations were measured by Sandwich Enzyme immuno-assay system (ELISA) by the standard kit provided by ERBA LISA in which a monoclonal antibody to prolactin is immobilized on the micro-wells. A purified polyclonal prolactin antiserum conjugated with the enzyme Horseraddish peroxidase is used to detect prolactin.
Statistical analysis
The mean (x) and standard deviation (SD), Standard error of mean (SEM) were calculated. The p-value was calculated using student’s t-test. (which considered significant when p< 0.01 and highly significant at p<0.001)
Result and Discussion
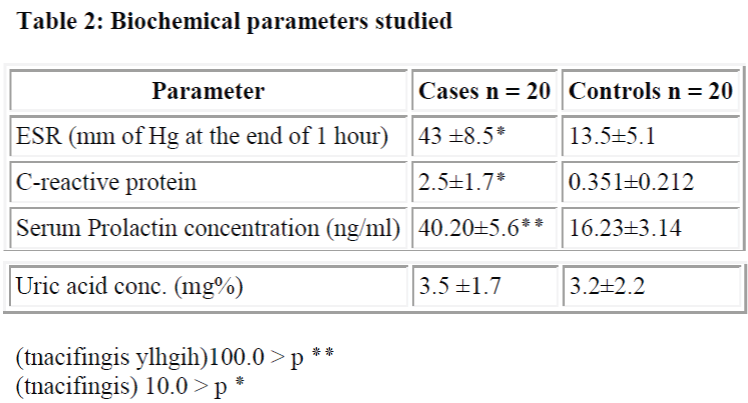
In our studies, serum prolactin concentrations in 20 female patients of rheumatoid arthritis were significantly increased than in normal healthy controls Mateo L, Nolla JM, Bonnin MR, Navarro MA, Roig-Escofet [5] similarly found raised serum prolactin levels up to 249±162mU/l (p = 0.0015) in men with rheumatoid arthritis than the normal healthy adults. McMurray RW, Allen SH, Pepmueller PH, Keisler D, Cassidy JT [6] moreover shown elevated serum prolactin levels in-patients with antinuclear antibody positive juvenile rheumatoid arthritis which is a variant of rheumatoid arthritis occurring in juvenile age group. However, the exact source of increased prolactin cannot be commented upon from our study.
Fuxe K, Hökfelt T, Eneroth P, Gustafsson JA, and Skett.P.[12] first reported the prolactin immuno-reactivity in hypothalamic axon terminals. Prolactin immuno-Prolactin in arthritis reactivity was subsequently found in the telencephalon, hippocampus, amygdala, septum [13], caudate putamen [14,15], brain stem [13,15], cerebellum [1], spinal cord [1,15], choroid plexus and the circumventricular organs [1]. “Lymphocytes can be a source of prolactin as well” [16]. This fact was accepted based on various feasible studies [1,16]. Prolactin mRNA was found in immune-competent cells from thymus and spleen as well as peripheral lymphocytes [1]. This mRNA releases a bioactive prolactin that is similar to pituitary prolactin [1,17,18,19]. Not only is an immunoreactive 22-kDa prolactin found in murine and human immune-competent cells, but size variants of prolactin have been described as well [20,21]. The control of pituitary prolactin secretion differs from that of lymphocytic origin. There is evidence that lymphocytes contain dopamine receptors that may be involved in the regulation of lymphocytic prolactin production and release [22]. There are studies of pharmacological characterization of lymphocytic dopamine receptors. that rather than the classical D2 type receptors found on lactotrophs, both the D4 and D5 predominate on lymphocytes. Moreover, mRNA for the D1, D3, and D5 receptors has been identified in rat lymphocytes [1,23,24].
The question remains of the role for pituitary and lymphocytic prolactin in the immune response. In mice, acute skin allograft rejection was studied in which pituitary prolactin gene expression, bioassayable serum prolactin, immunoassayable serum prolactin and lymphocyte number were found to be elevated elevated Administration of bromocryptine, a D2 receptor agonist, or anti-lymphocytic serum diminishes circulating levels of prolactin in grafted animals and prolongs graft survival [26,27].
Because bromocryptine has little direct effect on lymphocytic prolactin secretion [26]. Such data suggested that pituitary prolactin may modulate the elaboration of lymphocytic prolactin and that suppression of pituitary prolactin is thus a requirement for graft survival [28]. Indeed, such a role for prolactin in transplant rejection warranted further investigation. The data indicate that suppression of prolactin levels decreased the graft rejection reaction i.e. immunity which in turn indicates the role of prolactin in increasing autoimmune response. Deficiency of circulating estrogens is implicated in rheumatoid arthritis [29] with the evidence for the reversal of symptoms with gonodal steroid therapy especially estrogen in artificially induced arthritis in mice [30]. No such study could be found in humans.
It is interesting to note that giving antiprolactin drugs to the patient of rheumatoid arthritis has improved the disease condition. In a study carried out by N.Erb, et al hormonal assay was done in the patient of rheumatoid arthritis. The patient had active synovitis with tender swollen joints, early morning stiffness and erythrocyte sedimentation rate (ESR) of 50 mm in the first hour. Serum prolactin was found to be increased. Prolactin antagonist cabergolin 500 μgm/day for two months was given to the patient which resulted in decrease in the disease activity within two months. In humans, rheumatoid synovial T- cells produce prolactin. Also prolactin receptors are found on T, B and fibroblast like synovial cells [31]. Addition of prolactin to rheumatoid synovial cells in rats causes increased production of proteolytic enzymes causing cartilage destruction and increased production cytokines which indicates that prolactin injected in joints caused inflammation [31,32,33].
Our study indicates that there is a possible association between prolactin and development of rheumatoid arthritis. We are, however interested to explore the hormonal status and the efficacy of anti-prolactin drugs in remission of rheumatoid arthritis which may contribute for the treatment strategy.
References
- Marc E, Freeman BK, Anna L, György N. Prolactin: Structure, function, and regulation of secretion. Physiol Rev 2000; 80: 1523-1631.
- Bern HA, Nicoll CA. The comparative endocrinology of prolactin. Recent Prog Horm Res 1968; 24: 681-720.
- Chen HW, Weier H, Heiniger HJ, Huebner RI. Tumorigenesis in strain DW/J mice and induction by prolactin of the group-specific antigen of endogenous C- type RNA tumor virus. J Natl Cancer Inst 1972; 49: 1145-1153.
- Nagy E, Berczi I, Wren E, Asa SL, Kovacs K. Immunomodulation by bromocryptine. Immunopharmacology 1978: 6: 231-243.
- Mateo L, Nolla JM, Bonnin MR, Navarro MA, Roig-Escofet “High serum prolactin levels in men with rheumatoid arthritis”. D Journal of Rheumatology 1998; 25: 2077-2082.
- McMurray RW, Allen SH, Pepmueller PH, Keisler D, Cassidy JT. Elevated serum prolactin levels in children with juvenile rheumatoid arthritis and antinuclear antibody seropositivity. J Rheumatol. 1995; 22: 1577-1580.
- Orbach H, Shoenfeld Y. “Hyperprolactinemia and autoimmune diseases.” Autoimmun Rev. 2007 Sep;6(8):537-542. Epub 2006 Dec 1. Review.
- Brennan P, Silman A. Breast-feeding and the onset of rheumatoid arthritis.Arthritis Rheum 1994;37:808-813.
- Issel bacher, Braun wald,Wilson, Mertin,Fauci, Kasper; “Harrison’s Principles of Internal Medicine”1994 : 13th Ed., pp.1653-1655, McGraw Hill publication
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al.The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-324.
- Van der Heijde DMFM, van’t Hof MA, van Riel PLCM, Theunisse LM, Lubberts EW, van Leeuwen MA, et al. “Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score”Ann Rheum Dis 1990;49:916-920.
- Fuxe K, Hökfelt T, Eneroth P, Gustafsson JA, and Skett P. Prolactin-like immunoreactivity: localization in nerve terminals of rat hypothalamus. Science 1977,196: 899-900.
- Devito WJ. ” Distribution of immunoreactive prolactin in the male and female rat brain: effects of hypophysectomy and intraventricular administration of colchicine” Neuroendocrinology 1988,47: 284-289.
- Emanuele NV, Metcalfe L, Wallock L, Tentler J, Hagen TC, Beer CT, Martinson D, Gout PW, Kirsteins L, and Lawrence AM. Extrahypothalamic brain prolactin: characterization and evidence for independence from pituitary prolactin. Brain Res 1987,421: 255-262.
- Harlan RE, Shivers BD, Fox SR, Kaplove KA, Schachter BS, and Pfaff SW. Distribution and partial characterization of immunoreactive prolactin in the rat brain. Neuroendocrinology, 1989; 49: 7-22.
- Gala RR, and Shevach EM. Evidence for the release of a prolactin-like substance by mouse lymphocytes and macrophages. Proc Soc Exp Biol Med 1994;205: 12- 19.
- Dimattia GE, Gellersen B, Bohnet HG, and Friesen HG. A human B- lymphoblastoid cell line produces prolactin. Endocrinology 1988, 122: 2508-2517.
- Gellersen B, Dimattia GE, Friesen HG, and Bohnet HG. Phorbol ester stimulates prolactin release but reduces prolactin mRNA in the human B- lymphoblastoid cell line IM-9-P3. Mol Cell Endocrinol 1989,66: 153-161.
- Gellersen B, Dimattia GE, Friesen HG, and Bohnet HG. Regulation of prolactin secretion in the human B- lymphoblastoid cell line IM-9-P3 by dexamethasone but not other regulators of pituitary prolactin secretion. Endocrinology 1989, 125: 2853-2861.
- Sabharwal P, Glaser R, Lafuse W, Varma S, Liu Q, Arkins S, Kooijman R, Kutz L, Kelley KW, and Malarkey WB. Prolactin synthesized and secreted by human peripheral blood mononuclear cells: an autocrine growth factor for lymphoproliferation. Proc Natl Acad Sci USA 1992, 89: 7713-7716.
- Shah GN, Laird HE, and Russell DH. Identification and characterization of a prolactin-like polypeptide synthesized by mitogen-stimulated murine lymphocytes. Int Immunol 1991; 3: 297-304.
- Devins SS, Miller A, Herndon BL, O’Toole L, Reisz G. Effects of dopamine on T-lymphocyte proliferative responses and serum prolactin concentrations in critically ill patients. Crit Care Med , 1992, 20: 1644-1649.
- Bondy B, Ackenheil M, and Ruppert T. Spiperone binding in lymphocytes: part of a dopamine uptake system. Ann NY Acad Sci , 1992; 650: 221-225.
- Bondy B, De Jonge S, Pander S, Primbs J, and Ackenheil M. Identification of dopamine D4 receptor mRNA in circulating human lymphocytes using nested polymerase chain reaction. J Neuroimmunol 1996 ,71: 139-144.
- Shen GK, Montgomery DW, Ulrich ED, Mahoney KR, Zukoski CF. Up- regulation of prolactin gene expression and feedback modulation of lymphocyte proliferation during acute allograft rejection. Surgery 1992, 112: 387-394.
- Neidhart M. Bromocriptine has little direct effect on murine lymphocytes, the immunomodulatory effect being mediated by the suppression of prolactin secretion. Biomed Pharmacother 1997, 51: 118-125.
- Rosso Di San Secondo VEM, Fitch CA, Aniasi A, Close FT, Sirchia G, and Freeman ME. Bromocryptine prevents the immunosuppression induced in mice by anti-lymphocytic serum. Transplant Proc 1996, 28: 3193-3195.
- Martinelli GP, Liu H, Clarke WP, Heisenleder DJ, Knight RJ. Prolactin suppression enhances the effects of perioperative donor-specific blood transfusions on graft survival. J Surg Res 1996,64: 190-197.
- Wilder RL. Adrenal and gonadal steroid hormone deficiency in the pathogenesis of rheumatoid arthritis.J Rheumatol Suppl. ;1996: 44: 10-12.
- Subramanian S, Tovey M, Afentoulis M, Krogstad A, Vandenbark AA, Offner H. Ethinyl estradiol treats collagen-induced arthritis in DBA/1LacJ mice by inhibiting the production of TNF-alpha and IL-1beta.. Clin Immunol.: 2005;115:162-172.
- Erb N, Pace JV, Delamere JP, Kitas D. Control of unremitting rheumatoid arthritis by the prolactin antagonist cabergoline. British Journ of Rheumatology 2001; 40: 237-239.
- Nagafuchi H, Suzuki N, Kaneko A, Asai T, Sakane T. Prolactin locally produced by synovium infiltrating T lymphocytes induces excessive synovial cell functions in patients with rheumatoid arthritis. J Rheumatol1 999; 26: 1890-1900
- Neidhart M, Gay RE, Gay S. Prolactin and prolactin-like polypeptides in rheumatoid arthritis. Biomed Pharmacother1999; 53: 218-222