Research Article - Biomedical Research (2017) Volume 28, Issue 1
Sentinel bleeding after a pancreaticoduodenectomy: whether to perform a prophylactic embolization
Peng Song1*, Maoqiang Wang2, Ning Wu1, Feng Duan2, Dabin Xu3 and Fengyong Liu21Departments of Interventional Radiology, Hainan General Hospital, Hainan Province, 570100, PR China
2Departments of Interventional Radiology, Chinese PLA General Hospital, Beijing, 100853, PR China
3Department of Surgical Oncology, Chinese PLA General Hospital, Beijing, 100853, PR China
- *Corresponding Author:
- Peng Song
Departments of Interventional Radiology
Hainan General Hospital
PR China
Accepted on June 02, 2016
Abstract
To present a retrospective post-pancreaticoduodenectomy case series that had sentinel bleeding after pancreaticoduodenectomy and to evaluate the necessity and safety of prophylactic embolization. Fifteen post-pancreaticoduodenectomy patients with sentinel bleeding after pancreaticoduodenectomy were included in this study. Digital subtraction angiography was performed 6 hours after sentinel bleeding was detected; 8 patients received a prophylactic embolization, while 7 patients received digital subtraction angiography only. If a delayed massive hemorrhage occurred, intensive treatment such as a laparotomy or Transcatheter Angiographic Embolization (TAE) was performed. Fourteen (93.3%) patients’ angiographic findings were negative, and 1 patient had a pseudoaneurysm. All 8 prophylactic embolization patients were alive, and no delayed massive hemorrhage occurred. The delayed massive hemorrhage incidence with digital subtraction angiography was 42.8% (3 of 7), and 1 patient (14.3%) died because of a delayed massive hemorrhage. Compared with digital subtraction angiography, length of stay (p=0.048) and transfusion rates (p=0.002) decreased markedly in prophylactic embolization group. No serious complications related to prophylactic embolization were observed. Prophylactic embolization should be the treatment of choice for post-pancreaticoduodenectomy patients with sentinel bleeding, because it is safe and could reduce the postoperative length of stay and transfusion rate.
Keywords
Pancreaticoduodenectomy, Sentinel bleeding, Delayed massive hemorrhage, Prophylactic embolization, Pancreatic leak, Sepsis.
Introduction
The post-pancreaticoduodenectomy mortality rate has decreased markedly during the recent decades, while the morbidity rate still remains considerably high, ranging from 30% to 40% [1-3]. Hemorrhage is one of the most common causes of morbidity and mortality of postpancreaticoduodenectomy. Particularly, a delayed massive hemorrhage, which occurs 5 or more days after the operation, is associated with a high mortality rate [2,4]. Sentinel bleeding has been first introduced by Brodsky and Turnbullin 1991 [5] to describe minor blood loss via surgical drains or the gastrointestinal tract after pancreaticoduodenectomy. It is also one of the risk factors for a delayed massive hemorrhage. Angiography and Transcatheter Angiographic Embolization (TAE) have been recommended as a first-line diagnostic and treatment choice for a delayed massive hemorrhage after a pancreaticoduodenectomy [6], but the treatment strategy for sentinel bleeding is still controversial. Therefore, the aim of this study was to evaluate the efficacy of prophylactic embolization for sentinel bleeding following a pancreaticoduodenectomy.
Material and Methods
Patients
Fifteen patients received interventional treatments from April 2010 to August 2013 were recruited in our study. All patients had sentinel bleeding after a pancreaticoduodenectomy in a single center (The Chinese PLA General Hospital) (Table 1). Indications for surgery were pancreatic cancer (2 patients), chronic pancreatitis (1 patient), distal bile duct cancer (4 patients), duodenal papillary carcinoma (4 patients), ampullary carcinoma (1 patient), pancreatic cystadenoma (1 patient), and duodenal stromal tumors (2 patients). Among 15 patients, 8 patients received a prophylactic embolization procedure and 7 patients had conservative treatment. Whether a patient received prophylactic embolization or conservative therapy was dependent on angiographic images, surgeons’ advice and patient agreement. All patients were examined and treated as a part of routine care and had signed their informed consent.
| No. | Sex/Age | Pathology | Sentinel bleeding | Interval (POD) | Risk factors | Angiographic Finding | Prophylactic Embolization | Intervention after MDH (Bleeding site) | PRBC transfusions | LOS (POD) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/39 | Chronic pancreatitis | Drainage | 12 | Pancreatic leak and sepsis | Negative | HA and SA | No MDH occurred | 4 | 39 | Alive |
| 2 | M/48 | DST | Drainage | 8 | Pancreatic leak and sepsis | Negative | HA and SA | No MDH occurred | 6 | 60 | Alive |
| 3 | F/74 | DPC | Drainage | 7 | Pancreatic leak and sepsis | Negative | HA and SA | No MDH occured | 6 | 20 | Alive |
| 4 | M/72 | DPC | Drainage | 16 | Pancreatic leak | Negative | HA | No MDH occurred | 0 | 46 | Alive |
| 5 | F/74 | Pancreatic cystadenoma | Drainage | 17 | Pancreatic leak | Negative | DPA and SA | No MDH occurred | 0 | 26 | Alive |
| 6 | M/54 | Pancreatic ca. | GIT | 32 | Pancreatic leak | Negative | LGA and SA | No MDH occurred | 5 | 37 | Alive |
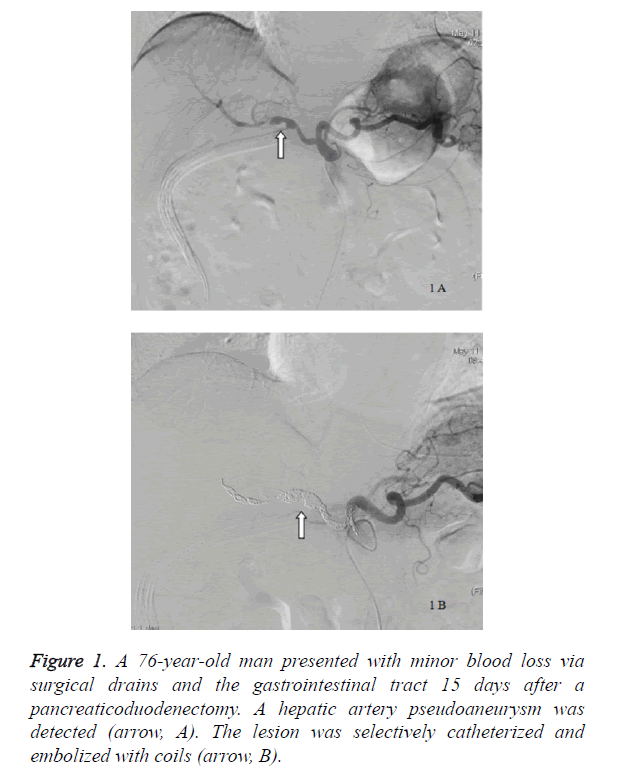
| 7 | M/76 | DPC | Drainage and GIT | 15 | Pancreatic leak and sepsis | HA PA | HA | No MDH occurred | 10 | 48 | Alive |
| 8 | F/54 | Pancreatic ca. | Drainage | 30 | Pancreatic leak and sepsis | Negative e | GDA remnant and SA | No MDH occurred | 0 | 38 | Alive |
| 9 | M/81 | DST | Drainage | 7 | Pancreatic leak and sepsis | Negative | NO | TAE (DPA and HA) | 58 | _ | Died |
| 10 | M/55 | DBDC | Drainage | 14 | Pancreatic leak | Negative | NO | No MDH occurred | 14 | 46 | Alive |
| 11 | M/68 | DPC | Drainage | 34 | Pancreatic leak and sepsis | Negative | NO | No MDH occurred | 6 | 50 | Alive |
| 12 | F/54 | DBDC | Drainage | 21 | Pancreatic leak | Negative | NO | Laparotomy (PV) | 17 | 98 | Alive |
| 13 | F/53 | Ampullary ca. | Drainage | 11 | Pancreatic leak | Negative | NO | Laparotomy (SA) | 12 | 69 | Alive |
| 14 | M/64 | DBDC | Drainage and GIT | 11 | Pancreatic leak | Negative | NO | No MDH occurred | 15 | 55 | Alive |
| 15 | M/57 | DBDC | Drainage and GIT | 13 | Pancreatic leak | Negative | NO | No MDH occurred | 11 | 38 | Alive |
| Note: POD: Postoperative Day; PA: Pseudo Aneurysm; GDA: Gastro Duodenal Artery; HA: Hepatic Artery; SA: Splenic Artery; DPA: Dorsal Pancreatic Artery; LGA: Left Gastric Artery; PRBC transfusion: Packed Red Blood Cell Transfusion; DBDC: Distal Bile Duct Cancer; DPC: Duodenal Papillary Carcinoma; DST: Duodenal Stromal Tumours; PV: Portal Vein; MDH: Massive Delayed Haemorrhage; Ca: Cancer, GIT, TAE. | |||||||||||
Table 1. Characteristics of patients who had sentinel bleeding after a pancreaticoduodenectomy.
Definitions
In our study, sentinel bleeding was defined as minor blood loss via surgical drains or the gastrointestinal tract after a pancreaticoduodenectomy [4,5,7]. This was further restricted to cases that did not have hemodynamic instability or a decrease in hemoglobin levels of more than 2 g/dL [7]. A delayed massive hemorrhage was defined as a life-threatening hemorrhage leading to hemorrhagic shock that occurred more than 5 days after a pancreaticoduodenectomy [1,4]. A pancreatic leak was defined by a 3 or more times greater amylase level in the drain fluid than that of the serum after the third postoperative day [1,7]. Sepsis was defined as a fever (>38.5°C) and a leucocytosis (WBC>15 × 109/L) based on a positive blood culture or a central vein catheter culture, or any combination of these, and/or pneumonia visualized on a chest x-ray [2,8,9].
Digital subtraction angiography and prophylactic embolization procedure
Digital subtraction angiography was performed within 6 hours after sentinel bleeding was detected in all patients. Briefly, the femoral artery was punctured, and a 4-Fr sheath was inserted. An abdominal aortography was obtained with a 4-Fr pigtail; thereafter, selective celiac arterial angiography and superior mesenteric arterial angiography were performed with a 4-Fr catheter. In the prophylactic embolization group, a prophylactic embolization was via a transarterial embolization of the branches of the celiac artery after an arterial angiography. The branches of the celiac artery included the hepatic artery (common hepatic artery, proper hepatic artery, right hepatic artery, and left hepatic artery), splenic artery, gastroduodenal artery, left gastric artery, and dorsal pancreatic artery. Briefly, microcatheters were inserted into the target arteries based on the surgeon’s treatment decision, and embolic materials were inserted. The embolic materials consisted of metal coils, gelfoam, and n-butyl cyanoacrylate-iodized oil mixture. The nbutyl cyanoacrylate-iodized oil mixture was prepared by hand that was obtained by mixing n-butyl cyanoacrylate and iodized oil in a 1:4 ratio. If a delayed massive hemorrhage occurred after a prophylactic embolization, intensive treatment such as a laparotomy or a TAE would be performed as appropriate.
Data analysis
The records of all patients with sentinel bleeding were then analyzed. Variables included the patient’s clinical characteristics, the presence of a pancreatic leak and sepsis, and diagnostic and therapeutic interventions related to bleeding. Furthermore, the occurrence of a delayed massive hemorrhage, the number of packed red blood cell units transfused, as well as the post-pancreaticoduodenectomy length of stay, complications, and outcomes, were extracted from the patients’ files. Statistical analysis was performed using the t test, rank-sum test, and χ2 test, when applicable. A P value of less than 0.05 was considered significant. Statistical analysis was performed by using SPSS version 11.5.
Results
Fifteen patients (10 men and 5 women) with a mean age of 61.5 years (range, 39 to 81 years) had sentinel bleeding after having a pancreaticoduodenectomy. All 15 patients (100%) had c post-pancreaticoduodenectomy pancreatic leak whereas 7 patients (46.7%) had sepsis. The average interval between sentinel bleeding and the pancreaticoduodenectomy was 16.5 ± 8.9 days. Eleven patients (73.3%) had minor blood loss via surgical drains, with 1 patient (6.7%) via the gastrointestinal tract and 3 patients (20%) via surgical drains and the gastrointestinal tract. The angiographic findings of 14 patients (93.3%) were negative, and a pseudoaneurysm was found in 1 patient (Figure 1).
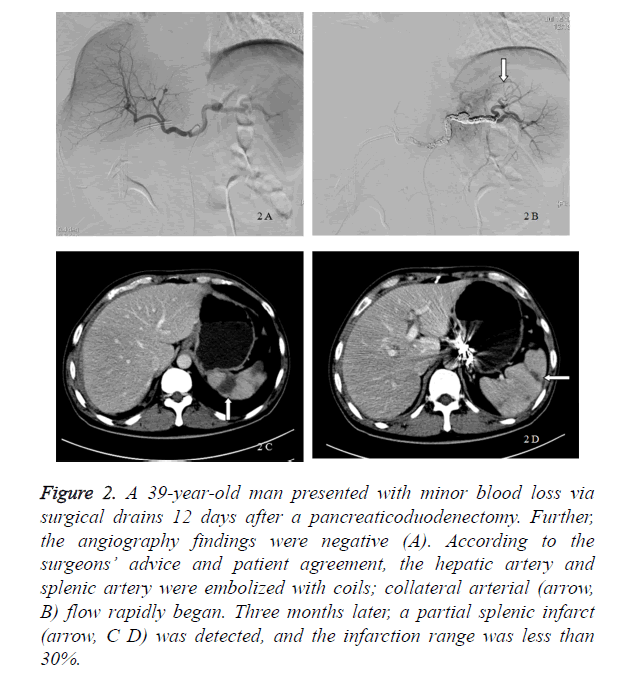
In our study, 8 patients received a prophylactic embolization, whereas 7 patients received a digital subtraction angiography only and were treated conservatively. The incidence of a delayed massive hemorrhage in the digital subtraction angiography group was 42.8% (3 of 7 patients), and 1 patient (14.3%) died because of a delayed massive hemorrhage. Six patients were alive, and the average postpancreaticoduodenectomy length of stay was 59.33 ± 21.57 days. If a delayed massive hemorrhage occurred, a laparotomy (2 patients) and TAE (1 patient) were performed. The bleeding sites included the portal vein, splenic artery, dorsal pancreatic artery, and hepatic artery. The patients underwent transfusion with a median of 14 U of packed red blood cells (Table 2). In the prophylactic embolization group, all 8 patients were alive and had no delayed massive hemorrhage after the interventional procedures. Their average postpancreaticoduodenectomy length of stay was 39.25 ± 12.57 days. The patients underwent transfusion with a median 4.5 [6] U of packed red blood cells. Compared with the digital subtraction angiography group, the postpancreaticoduodenectomy length of stay (p=0.048) and red blood cell transfusion volume (p=0.002) decreased markedly in the prophylactic embolization group (Table 2). The embolized arteries included the hepatic artery (5 patients), splenic artery (6 patients), dorsal pancreatic artery (1 patient), left gastric artery (1 patient), and gastroduodenal remnant (1 patient). Complications related to the prophylactic embolization procedures were rare. Five patients who received a hepatic artery embolization had a transient increase in aspartate aminotransferase and alanine aminotransferase levels because of hepatic ischemia. A partial splenic infarct was seen in the patients who received a splenic artery embolization, and the infarction range was less than 30%. Liver necrosis or splenic abscess was not detected in any of these patients (Figure 2).
| (n=8) | DSAG (n=7) | Significance (p value) | |
| Packed red blood cell transfusion (median, interquartile range) |
4.5 (6) | 14 (6) | 0.002 |
| post- pancreaticoduodenectomy length of stay (mean ± SD) |
39.25 ± 12.57 | 59.33 ± 21.57* | 0.048 |
| Mortality rate | 0 | 0.143 | 0.467 |
| Morbidity rate with a delayed massive hemorrhage | 0 | 0.428 | 0.077 |
| *1 patient died, n=6 Note: PEG: Prophylactic Embolization Group; DSAG: Digital Subtraction Angiography Group; DSA: Digital Subtraction Angiography. |
|||
Table 2. Comparison of parameters between the prophylactic embolization group and DSA group.
Figure 2. A 39-year-old man presented with minor blood loss via surgical drains 12 days after a pancreaticoduodenectomy. Further, the angiography findings were negative (A). According to the surgeons’ advice and patient agreement, the hepatic artery and splenic artery were embolized with coils; collateral arterial (arrow, B) flow rapidly began. Three months later, a partial splenic infarct (arrow, C D) was detected, and the infarction range was less than 30%.
Discussion
The present retrospective study has demonstrated that the use of prophylactic embolization may be beneficial for the treatment of sentinel bleeding after pancreaticoduodenectomy in Chinese patients. Prophylactic embolization may reduce the volume of blood transfusion as well as the post-operational hospital stay. After a pancreaticoduodenectomy, a hemorrhage is reported in 5-16% of patients and is associated with high morbidity and mortality [4]. An early postoperative hemorrhage is mostly caused by technical failure, poor primary hemostasis, or insufficient management of coagulation disorders [2,8,10]. The most common causes of a delayed hemorrhage, occurring 5 or more days after a pancreaticoduodenectomy, were erosive bleeding of skeletonized vessels and pseudoaneurysm rupture [1,2,4,6,9]. A delayed hemorrhage is always dramatic and accompanied by a sudden drop in blood pressure and hemoglobin levels. An accurate diagnosis and efficient treatment are crucial. Finding and suturing the bleeding site could be achieved by performing a second laparotomy [1,6,8,10]. However, hemodynamic instability and the burden of general anesthesia would aggravate the patient’s condition and could be life threatening. Furthermore, massive adhesions in the upper abdomen would make it difficult to fully expose the bleeding site [11]. With the development of interventional radiology, transcatheter angiographic embolization (TAE) has been suggested as a firstline diagnostic and treatment choice, because it is minimally invasive, time saving, and effective [1,4,6,8,12-14].
Sentinel bleeding, used to be defined as minor blood loss via surgical drains or the gastrointestinal tract with an asymptomatic interval between bleeding and hemorrhagic shock, has now been reported to precede with a delayed massive hemorrhage in 25 to 100% of cases [2,4-7,10,12]. Unlike an early postoperative minor hemorrhage that occurred within the first few days post-operationally and usually does not require any intervention, sentinel bleeding has been regarded as a prelude to a delayed massive hemorrhage [4,9]. Hence it should not be neglected. Time is the key factor that influences patient survival with a delayed hemorrhage. In our study, if sentinel bleeding was detected, interventional treatment was immediately performed within 6 hours. Moreover, previous studies have suggested the use of low molecular weight heparin (LMWH) to prevent post-operational embolism [15]. Whereas other studies also suggested that heparin may increase the rate for post-surgical hemorrhage [16]. In our study, no patients have received low molecular weight heparin for prevention of post-operational embolism. Therefore, LMWH related hemorrhage could be ruled out in our study.
In accordance with the literature, a pancreatic leak and sepsis are independent risk factors for post-pancreaticoduodenectomy bleeding [6,8,17]. A theory pertaining to local sepsis that was caused by a pancreatic leak has been accepted as the main cause for a delayed massive hemorrhage [1,14,18]. Usually the vasculature is injured by skeletization with a lymphadenectomy that is performed during a pancreaticoduodenectomy, and a pancreatic leak or an intra-abdominal abscess could erode the anastomosis site or vascular wall. This injury mechanism may result in a delayed hemorrhage in the form of arterial pseudoaneurysm formation or arterial bleeding [1,5]. The importance of early detection of sentinel bleeding in the prevention or intervention of a delayed massive hemorrhage has been widely recognized, but the treatment strategy for sentinel bleeding is still controversial. According to the theory, surgeons have suggested prophylactic embolization of the branches of the celiac artery in order to prevent a massive delayed hemorrhage. In our study, 8 patients having pancreatic leak including 5 had sepsis received a prophylactic embolization. All were alive, and no delayed massive hemorrhage occurred. Compared with the patients in the digital subtraction angiography group, the post-pancreaticoduodenectomy length of stay (p=0.048) and packed red blood cell transfusion volume (p=0.002) in the prophylactic embolization group were significantly reduced. Meanwhile, the incidence of a massive delayed hemorrhage did not approached statistical significance (p=0.077) (Table 2), as the number of cases was limited.
Embolization ischemia is one of the most common complications of TAE [7,10]. In our study, hepatic ischemia after a hepatic artery embolization caused a dramatic increase in aspartate aminotransferase and alanine aminotransferase levels, and all patients had an uneventful recovery. Meanwhile, no hepatic infarct occurred after a hepatic artery embolization. The low incidence of hepatic infarct after a hepatic artery embolization was because of portal venous flow and the existence of collateral arterial flow. Prior to embolization of the common or proper hepatic artery, superior mesenteric arterial angiography should be performed in order to identify portal venous patency and avoid any potential complications. If the portal vein was blocked, covered stents should be used in order to maintain hepatic arterial patency while achieving hemostasis [7,10,19]. A splenic infarct was the most common complication after a splenic artery embolization. In our study, metal coils were used to embolize the splenic artery, and collateral arterial flow rapidly began. The limited splenic infarction range after a splenic artery embolization was due to the existence of collateral arterial flow (Figure 2).
Re-bleeding may be caused by insufficient embolization or persistent erosion of the vessels [18,20]. If hemostasis was not adequately achieved by a TAE, then a laparotomy could be performed. However, if re-bleeding occurred several days after a TAE, it was mainly related to persistent erosion of a pancreatic leak, a laparotomy or second TAE should be the treatment of choice. Elimination of the risk factors such as pancreatic leak and sepsis is important in order to avoid re-bleeding [6,8,17,20]. In our study, after a sufficient prophylactic embolization, 8 patients received sufficient percutaneous drainage of a pancreatic leak after a pancreaticoduodenectomy, and none had a delayed massive hemorrhage or re-bleeding.
This study also had several limitations. First, our retrospective study design in nature would bring in biases in the sample selection. Second, our sample size was too small to detect the differences in the mortality and mobility between embolization and group. Moreover, a multi-variable regression could not be appropriately performed due to our small sample size. Therefore, confounding factors including patients characteristics affecting post-surgical bleeding could not be discussed. In conclusion, our study demonstrated that a prophylactic embolization should be considered as the treatment of choice for patients with sentinel bleeding after a pancreaticoduodenectomy. It is safe and it can markedly decrease the post-pancreaticoduodenectomy length of stay and blood transfusion volume. Prospective studies or randomized control trials with larger sample size are warranted.
References
- Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg 2004; 199: 186-191.
- De Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, Obertop H, Gouma DJ. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg 2005; 241: 85-91.
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447-458.
- Treckmann J, Paul A, Sotiropoulos GC, Lang H, Ozcelik A, Saner F, Broelsch CE. Sentinel bleeding after pancreaticoduodenectomy: a disregarded sign. J Gastrointest Surg 2008; 12: 313-318.
- Brodsky JT, Turnbull AD. Arterial hemorrhage after pancreatoduodenectomy. The ‘sentinel bleed’. Arch Surg 1991; 126: 1037-1040.
- Zhang J, Zhu X, Chen H, Qian HG, Leng JH, Qiu H, Wu JH, Liu BN, Liu Q, Lv A, Li YJ, Zhou GQ, Hao CY. Management of delayed post-pancreaticoduodenectomy arterial bleeding: interventional radiological treatment first. Pancreatology 2011; 11: 455-463.
- Tien YW, Wu YM, Liu KL, Ho CM, Lee PH. Angiography is indicated for every sentinel bleed after pancreaticoduodenectomy. Ann Surg Oncol 2008; 15: 1855-1861.
- Koukoutsis I, Bellagamba R, Morris-Stiff G, Wickremesekera S, Coldham C, Wigmore SJ, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Haemorrhage following pancreaticoduodenectomy: risk factors and the importance of sentinel bleed. Dig Surg 2006; 23: 224-248.
- Rajarathinam G, Kannan DG, Vimalraj V, Amudhan A, Rajendran S, Jyotibasu D, Balachandar TG, Jeswanth S, Ravichandran P, Surendran R. Post pancreaticoduodenectomy haemorrhage: outcome prediction based on new ISGPS Clinical severity grading. HPB (Oxford) 2008; 10: 363-370.
- Robinson K, Rajebi MR, Zimmerman N, Zeinati C. Post-pancreaticoduodenectomy hemorrhage of unusual origin: treatment with endovascular embolization and the value of preoperative CT angiography. J Radiol Case Rep 2013; 7: 29-36.
- Schäfer M, Heinrich S, Pfammatter T, Clavien PA. Management of delayed major visceral arterial bleeding after pancreatic surgery.HPB (Oxford) 2011; 13: 132-138.
- Tsirlis T, Vasiliades G, Koliopanos A, Kopanakis N, Katseli A, Tsipras H, Margaris H. Pancreatic leak related hemorrhage following pancreaticoduodenectomy. A case series. JOP 2009; 10: 492-495.
- Blanc T, Cortes A, Goere D, Sibert A, Pessaux P, Belghiti J, Sauvanet A. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg 2007; 194: 3-9.
- Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, Tanaka. Coil embolization ofbleeding visceral pseudoaneurysms following pancreatectomy:the importance of early angiography. Arch Surg 1998; 133: 1099-1102.
- Ciccone MM, Cortese F, Corbo F, Corrales NE, Al-Momen AK, Silva A, Zito A, Pinto M, Gesualdo M, Scicchitano P. Bemiparin, an effective and safe low molecular weight heparin: a review. Vascul Pharmacol 2014; 62: 32-37.
- Hayashi H, Morikawa T, Yoshida H, Motoi F, Okada T, Nakagawa K, Mizuma M, Naitoh T, Katayose Y, Unno M. Safety of postoperative thromboprophylaxis after major hepatobiliary-pancreatic surgery in Japanese patients. Surg Today 2014; 44: 1660-1168.
- Wei HK, Wang SE, Shyr YM, Tseng HS, Tsai WC, Chen TH, Su CH, Wu CW, Lui WY. Risk factors for post-pancreaticoduodenectomy bleeding and finding an innovative approach to treatment. Dig Surg 2009; 26: 297-305.
- Otah E, Cushin BJ, Rozenblit GN, Neff R, Otah KE, Cooperman AM. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg 2002; 137: 55-59.
- Lee HG, Heo JS, Choi SH, Choi DW. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy.World J Gastroenterol 2010; 16: 1239-1244.
- Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, Kaifi J, Schurr PG, Bubenheim M, Nolte-Ernsting C, Adam G, Izbicki JR. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 2007; 246: 269-280.