- Biomedical Research (2015) Volume 26, Issue 2
Screening of hepatoprotective activity of Madhuca longifolia bark on D-Galactosamine induced hepatotoxicity in rats.
Samaresh Pal Roy1,2*, T. Kannadasan2, Ramji Gupta1,21Department of Pharmacology, Shree Dhanvantary Pharmacy College, Kim, Surat, Gujarat, India
2Department of Pharmacology, Anna University, Chennai, Tamilnadu, India
- *Corresponding Author:
- Samaresh Pal Roy
Department of Pharmacology
Shree Dhanvantary Pharmacy College
Kim, Surat, Gujarat
India
Accepted date: February 16, 2015
Abstract
In this present investigation the ethanolic extract of Madhuca longifolia bark (EEMLB) was screened for hepatoprotective activity against D-galactosamine (d-GalN) induced hepatiotoxicity in rats. Wistar rats were divided into five groups (n=6). Hepatotoxicity in rats was achieved by intraperitoneal injection of 400mg/kg of d-GalN for five days. Silymarin (100mg/kg) was given as reference standard. The degree of protection against liver toxicity was determined by measuring the serum biochemical parameters viz. SGPT (serum alkaline phosphatase), SGOT (serum glutamine oxaloacetate transaminase), ALP (alkaline phosphatase), total cholesterol and bilirubin (Total and Direct). The result shows that d-GalN has enhanced the levels of SGPT, SGOT, ALP, total cholesterol and bilirubin. Pretreatment with EEMLB (200mg/kg B. wt. and 400 mg/kg B. wt.) brought back the altered levels of biochemical markers to the near normal levels. Histopathological studies also confirmed the hepatoprotective activity of these extracts when compared with d-GalN treated groups. It can be concluded from the result that the extract of Madhuca longifolia bark possesses hepatoprotective activity against D-GalN induced hepatotoxicity in rats.
Keywords
Madhuca longifolia bark, Hepatoprotective, D-galactosamine
Introduction
Hepatitis induced by D-galactosamine shows many metabolic and morphological aberrations in the livers of experimental animals similar to those observed in human viral hepatitis [1]. GalN hepatitis is induced by a multiple step mechanism [2]. In particular, the peroxidation of endogenous lipids has been shown to be a major factor in the cytotoxic action of GalN [3]. GalN-induced oxidative damage is generally attributed to the formation of the highly reactive hydroxyl radical (OHº), stimulator of lipid peroxidation and source for destruction and damage to the cell membrane [4].
In the absence of reliable modern hepatoprotective drugs, there are a number of traditional medicines recommended for treatment of liver diseases. Many herbs reportedly possess hepatoprotective activity such as Silybum marianum [5], Tridax procumbens [6], Strychnos potatorum [7], Andrographis paniculata [8], Picrorhiza kurroa [9], and Aquilegia vulgaris [10].
Madhuca longifolia belonging to the family Sapotaceae is an indigenous plant found largely in the central and north Indian plains and forests [11]. Commonly known as Mahua, it is used in the treatment of epilepsy, and inflammations. Presence of a genuine sapogenol protobasic acid and prospagenol in the seed kernels of Madhuca longifolia was reported by Yosioka [12]. Analgesic activity of the alcoholic extract was reported [13] while antihperglycemic activity [14] and anti-inflammatory activity [15] of the extract of Madhuca longifolia bark was also studied. The plant possesses remarkable antioxidant and hepatoprotective potential against carbon tetra chloride and dgalactosamine induce hepatic injury [16,17].
This study aimed to conduct a scientific experiment using the ethanolic extract of Madhuca longifolia bark at different doses from previous report to investigate its hepatoprotective activity and possible mechanism in Dgalactosamine induced rat hepatic injury.
Materials and Methods
Chemicals
D-Galactosamine (d-GalN) was purchased from Merck India Ltd., Mumbai, India. 5,5-dithiobis-2-nitrobenzoic acid was obtained from Sisco Research Laboratories Pvt. Ltd., Mumbai. Ecoline assay kits for serum aspartate aminotransferase (ASAT), alanine amino transaminase (ALAT), alkaline phosphatase (ALP), total Cholesterol (TC), Total & Direct bilirubin (TB) were obtained from Merck Ltd., Ambemath, India and Silymarin from Ranbaxy India Ltd., New Delhi. All the other chemicals used were of analytical grade.
Collection & Extraction
Leaf samples of the plant Polyalthia longifolia were collected from Bardoli, Surat, Gujarat, during January 2013 and was authenticated by Dr. Bimal Shah. The leaves of the plant were shade dried at room temperature and were then pulverized. The coarse powder obtained was successively extracted with various organic solvents in the increasing order of their polarity (petroleum ether, chloroform, ethanol & water) in a soxhlet extractor for a period of 24 – 28 hours. The extracts were then concentrated to dryness in a rotavapor under reduced pressure and controlled temperature. The ethanol extract yielded a brown semi-solid (16g).
Animals
Both sex Wistar rats (150 – 200g) were selected for the study. They were housed in polypropylene cages in an air-conditioned area at 22°C ± 3°C and 59 to relative humidity with 12-hour light & dark cycle. All the animals had free access to standard diet and clean water ad libitium. The experiments were conducted according to the Institutional Animal Ethics Committee (IAEC) regulations approved by the Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Experimental protocol
The rats were grouped randomly into five groups, each containing six animals. Group I, the negative control group, received the vehicle (normal saline). Group II served as positive control group and received the vehicle (normal saline). Group III was treated with standard drug silymarin at 100 mg /kg body weight. Group IV and V were treated with plant extract at the dose levels of 200 and 400 mg/kg body weight respectively for five days [18].
Group-I : Normal Saline (1ml/kg, p.o)
Group-II : d-GalN (400mg/kg, i.p)
Group-III : Silymarin (100mg/kg p.o.)+d-GalN (400mg/kg, i.p)
Group-IV : EEMLB (200 mg/kg p.o.) + d-GalN (400mg/kg, i.p)
Group-V : EEMLB (400mg/kg p.o.) + d-GalN (400mg/kg, i.p)
On the fifth day of the treatment, animals of all groups except group I received a single dose of d-GalN in distilled water at 400 mg/kg body weight intraperitoneally after two hours of their respective treatment. After 24 hours of d-GalN administration blood was collected retroorbitally under light ether anesthesia. Immediately, after blood withdrawal all groups were sacrificed. Liver samples were also collected for histological and biochemical estimations. The blood samples were allowed to clot for 30 – 40 minutes. Serum was separated by centrifugation at 37°C and was used for estimation of various biochemical parameters like SGOT, SGPT, ALP, total cholesterol, total & direct bilirubin [19-22].
Histological studies
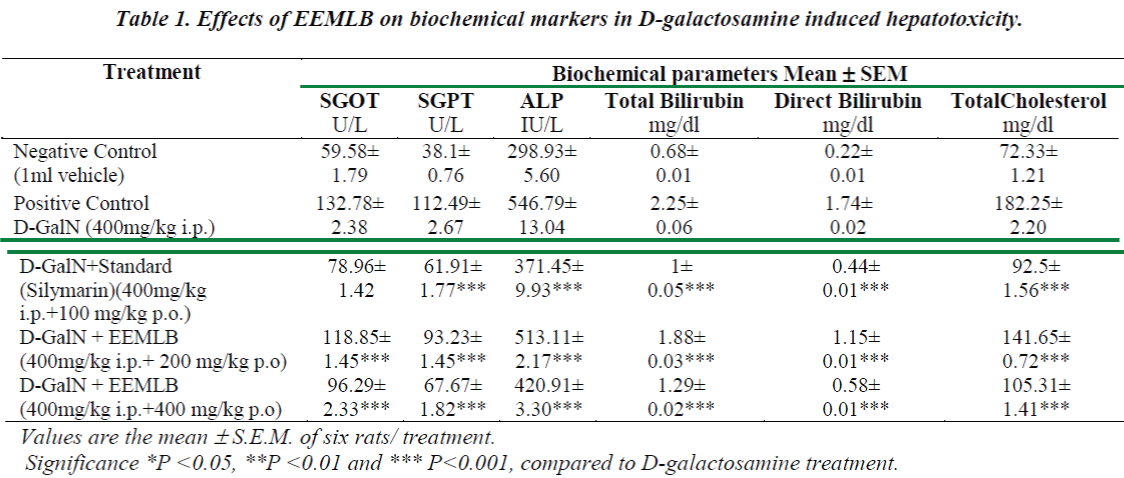
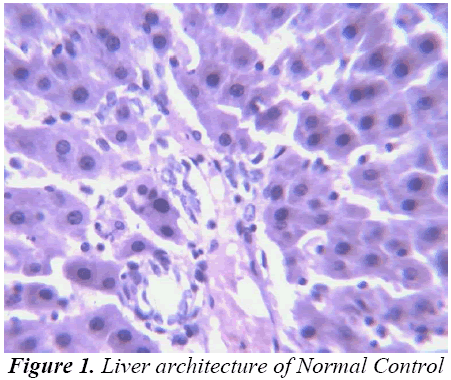
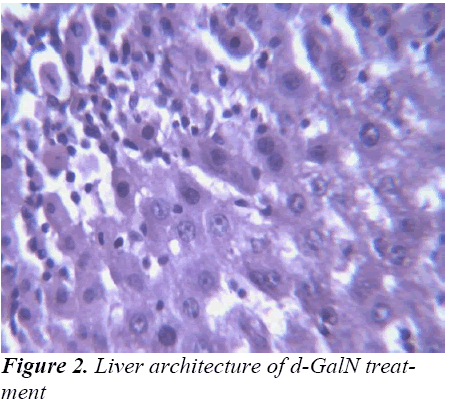
Small pieces of liver fixed in 10% buffered neutral formalin were processed for embedding in paraffin. Sections of 5–6 μm thickness were stained with hematoxylin and eosin and examined for histopathological changes (200×) under a compound microscope [23].
Data analysis
Quantitative data were expressed as mean ± S.E. and all statistical comparisons were made by means of one-way ANOVA test followed by Tukey’s test. P-Values less than 0.05 were considered statistically significant while P-values less than 0.01 were considered extremely significant.
Results
Biochemical observations
Administration of d-GalN resulted in a significant rise in the levels of SGPT, SGOT, ALP, Total Cholesterol and Bilirubin (Total and Direct) when compared to the vehicle treated group (Group-I). The extract of EEMLB treatments significantly (P<0.001) reversed the levels of elevated biochemical parameters in dose dependent manner (Group IV & V). The results indicated that the effect of test extract on biochemical markers was found to be almost comparable than the reference standard, Silymarin (Group III). The results are shown in (Table-1).
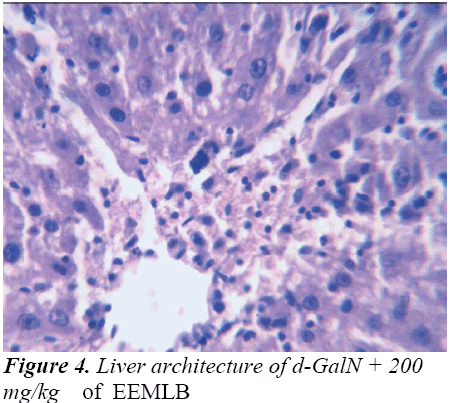
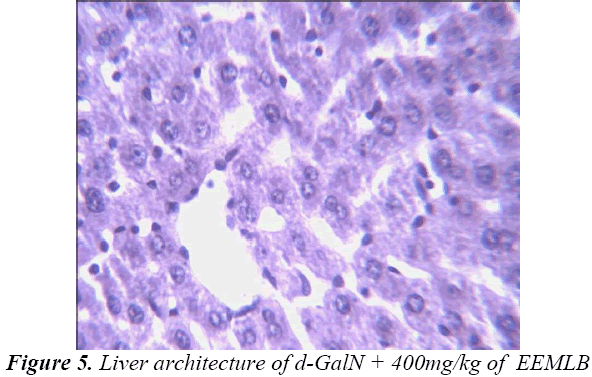
Histopathological Observation in D-Galactosamine induced hepatotoxicity
Discussion
Any disease/disorder is associated with cell injury due to the generation of free radicals like superoxide anion (O2·), NO radical, NOO·, OH· and H2O2 radical. Free radicals damage the cell membrane and cellular constituents like DNA etc. Though the free radicals are generated even in health, human beings possess the inbuilt natural mechanism to scavenge the generated free radicals. The inbuilt scavenging systems constitute the glutathione, superoxide dismutase (SOD), catalase. But during prolonged stressful conditions the free radicals produced cannot be handled by our inbuilt mechanism alone.
Thus released free radicals react with the membrane polyunsaturated fatty acid (PUFA) and oxidise them to lipid peroxides. This lipid peroxidation damages membrane protein as well as the lipids. Thereby, the integrity of the membrane is lost. Therefore it is considered that the extent of lipid peroxidation is directly proportional to cell damage. In addition, the free radicals may also attack DNA and causes tissue damage. Galactosamine administration in rats disrupts the membrane permeability of the plasma membrane causing leakage of the enzymes from the cell, which leads to elevation in levels of serum enzymes (Mitra et al., 2000). It is apparent that the levels of SGPT, SGOT, ALP, total cholesterol, and total and direct Bilirubin increased significantly in group treated with d- GalN comparing to normal control and it is an obvious indication of hepatic insult [24-26].
Study of any herbal medicine becomes more significant when it ameliorates some diseases conditions. In the present investigation, hepatoprotective effects of the ethanolic extract of Madhuca longifolia bark was studied based on d-GalN induced liver hepatitis. The hepatoprotective effect of Madhuca longifolia has been shown in earlier studies. In our study the rise in SGOT, SGPT, ALP, and bilirubin levels induced by D-galactosamine administration was significantly reduced by EEMLB pre-treatment suggesting that its hepatoprotective activity might be due its effect against cellular leakage and loss of functional integrity of the cell membrane in hepatocytes.
Histopathological studies also support the protective effects of the plant. Flavonoids, a polyphenolic derivative could be the major contributory factor in hepatoprotective activity [27,28]. It seems the protective activity of the plant may be due to strengthening the inbuilt antioxidant system and the antioxidant principles in the plant. However, further studies are needed to completely establish the mechanism of hepatoprotective effect of the plant.
Conclusion
Based on the present study, it can be concluded that ethanolic extract of Madhuca longifolia bark have potent hepatoprotective activity in a dose dependent manner. Further isolation of active principles will be advantageous to produce novel bioactive constituents from these extracts, which may possess more significance in the treatment of liver diseases, and to elucidate its exact mechanism of action. Attempts are being made to isolate and characterize the active principle to which the hepatoprotective activity can be attributed.
Acknowledgements
The authors express their deep sense of gratitude to the entire fraternity of Shree Dhanvantary Pharmacy College for their constant encouragement and support. We also express thanks to Shree Dhanvantary Pharmaceutical Analysis and Research Centre (SDPARC) for their kind cooperation and technical assistance.
References
- Gu CH, Cao R, Wang GX. Chung Hua Nei Ko Tsa Chih 1991; 30:17. ??
- Black DD, Tso P, Weidmann S, Sabesin SM. J Lipid Res 1983; 24:977.??
- Sakaguchi S, Yokota K. Pharmacol Toxicol 1995; 77:81.??
- Barry H, Gutteridge JMC. In: Barry H, editor. Free radicals in biology and medicine. Oxford: Clarendon Press, 1989; 254-255.
- Flora, K., Hahn, M., Rosen, H., Benner, K., Milk thistle (Silybum marianum) for the therapy of liver disease. The American J of Gastroenterol 1998; 93: 139–143.
- Ravikumar, V., Shivashangari, K.S., Devaki, T.,. Hepatoprotective activity of Tridax procumbens against d galactosamine/lipopolysaccharide-induced hepatitis in rats. J of Ethnopharmacol 2006; 101: 55–60.
- Sanmugapriya, E., Venkataraman, S., Studies on hepatoprotective and oxidant actions of Strychnos potatorum Linn. seed on CCl4 induced acute hepatic injury in experimental rats. Jo of Ethnopharmacol 2006;105: 154–160.
- Pramyothin, P., Chirdchupunsare, H., Rungsipipat, A., Chaichantipyuth, C., Hepatoprotective activity of Thunbergia laurifolia Linn. extract in rats treated with ethanol: in vitro and in vivo studies. J of Ethnopharmacol 2005; 102: 408–411.
- Saraswat, B., Visen, P.K.S., Patnaik, G.K., Dhawan, B.N., Ex vivo and in vivo investigations of Picrorhiza kurroa in an alcohol intoxication model in rats. J of Ethnopharmacol 1999; 66: 263–269.
- Liebert, J.J., Matlawska, I., Bylka, W., Murias, M.,. Protective effect of Aquilegia vulgaris on APAP induced oxidative stress in rats. J of Ethnopharmacol 2005;97: 351–358.
- Nadkarni AK. Indian Materia Medica. Bombay Popular Book Dept 1954; 3(1): 181
- Yosioka I, Indada A, Kitagawa I. Structures of a genuine sapogenol protobasis acid and a prosapogenol of seed kernels of Madhuca longifolia. Tetrahedron 1974; 30(6): 707-714.
- Dinesh Chandra. Analgesic effect of aqueous and alcoholic extract of Madhuca longifolia (Koeing). Indian J Pharmacol 2001; 33: 108-111
- Akash P Dahake, Chirantan S. Chakma, Rita C.Chakma, Prashant Bagherwal. Antihyperglycemic activity of methanolic extract of Madhuca longifoliabark. Diabetol Croatica. 2010, 39(1),1-8.
- Devendra Shirode, Samaresh Pal Roy, Tushar Patel, S. RamachandraSetty, S. V. Rajendra, Antiinflammatory activity of 70% ethanolic extract of Albizzia lebbeck leaves andMadhuca longifoliabark. Int. J. Pharmacol. Biol. Sci. Vol. 2(3) 2008, 127-130
- Samaresh Pal Roy,Devendra Shirode, Tushar Patel, C.S.Shastry, N. Gheewala, Goutam Sonara, S Ramachandra Setty and S.V Rajendra, Antioxidant and Hepatoprotective activity of Madhuca longifolia(koenig) bark against CCl4 - induced hepatic injury in rats: in vitro and in vivo studies Res J Pharm Bio Chem Sci. 2010;1(1):1-10.
- Samaresh Pal Roy, Ramji Gupta, T Kannadasan. Hepatoprotective activity of ethanolic extract of Madhuca longifolialeaves on D-galactosamine induced liver damage in rats. J. of Chem. Ph. Sci. 2012; 4: 205-09. 18.
- S.Shyamal, LathaP.G,Shine V.J,Suja S.R,Rajasekharan, S ,GangaDevi T,Hepatoprotective effects of ittosporum neelgherrense Wight&Arn.,a popular Indian ethnomedicine, J of Ethnopharmacol 2006; 107: 151-55
- Bergmeyer, H.U., IFCC methods for the measurement of catalytic concentrationsof enzymes: part 3. IFCC method for alanine aminotransferase (l-alanine: 2- oxoglutarate aminotransferase). Clin Chem Acta 1980; 105:147–154.
- Bergmeyer, H.U., Bowes Jr., G.N., Hørder, M.J., Moss, D.W., Provisional recommendations on IFCC methods for the measurement of catalytic concentrationsof enzymes. Part 2. IFCC method for aspartate aminotransferase. Clin Chem Acta 1976; 70; F19–F42.
- Kind, P.R., King, E.J., Estimation of plasma phosphatise by determinationof hydrolysed phenol with amino-antipyrine. J of Cli Path 1954; 7; 322–326.
- Walters, M.I., Gerarde, H.W., An ultramicromethod for the determinationof conjugated and total bilirubin in serum or plasma. Microchem J 1970; 15: 231.
- Lin, C.C., Shieh, D.E., Yen, M.H., Hepatoprotective effect of the fractions of Ban-zhi-lian on experimental liver injuries in rats. J of Ethnopharmacol 1997; 56; 193–200
- Ahmad, A., Pillai, K.K., Najmi, A.K., Ahmad, S.J., Pal, S.N., Balani, D.K.,.Evaluation of hepatoprotective potential of jigrine post-treatment against thioacetamide induced hepatic damage. J of Ethnopharmacol 2002; 79: 35–41.
- Friedman, L.S., Martin, P., Munoz, S.J., Liver function tests and the objective evaluation of the patient with liver disease. In: Zakim, D., Boyer, T. (Eds.), Hepatology. W.B. Saunders Company, London 1996; 791-833.
- Vimal, V., Devaki, T., Hepatoprotective effect of allicin on tissue defense system in galactosamine/ endotoxin challenged rats. J of Ethnopharmacol 2004; 90: 151–154.
- Seevola D, Baebacini GM, Bona S. Flavonoids and hepatic cyclic monophosphates in liv-er injury. Boll Ins Sieroter. 1984; 63: 777-782.
- Wegner T, Fintelmann V. Flavonoids and bioactivity. Wein Med Wochem Sihr. 1911; 149: 241-247.