Research Article - Biomedical Research (2017) Volume 28, Issue 7
Screening of antibacterial, antituberculosis and antifungal effects of lichen Usnea florida and its thamnolic acid constituent
Meral Yılmaz Cankılıç1*, Nalan Yılmaz Sarıözlü1, Mehmet Candan1 and Funda Tay21Department of Biology, Faculty of Sciences, Anadolu University, Eskisehir, Turkey
2Department of Chemistry, Faculty of Science and Letters, Eskisehir Osmangazi University, Eskisehir, Turkey
- *Corresponding Author:
- Meral Yilmaz Cankilic
Faculty of Sciences, Department of Biology
Anadolu University, Turkey
Accepted date: December 06, 2016
Abstract
In the present study, the methanol, acetone and chloroform extracts of the lichen Usnea florida (L.) were tested for antibacterial, antifungal and antituberculosis potential. The disk diffusion method was used for the investigation of antimicrobial activity and the minimal inhibitory concentration was identified against thirteen species of bacteria, four species of yeasts, and eight species of fungi. Strong activity toward bacteria and yeasts was determined in all extracts. However, there was no strong activity toward filamentous fungi. Bioactive substances of lichen Usnea florida were demonstrated with the bio autography in thin layer chromatography. Thamnolic acid was found to be an active substance with 200-400 μg/ml concentration. As a result, the lichen may be used as a possible antimicrobial agent in various applications in the food industry and for the purpose of controlling different diseases.
Keywords
Lichen, Antimicrobial activity, Usnea florida, Thamnolic acid
Introduction
The lichens are symbiotic organisms consisting of fungi and green algae or cyanobacterium found in many ecosystems such as rock surfaces, poorly developed soils, on trees and shrubs. The results of this symbiosis, various unique extracellular secondary metabolites are produced [1].
Lichens and their secondary metabolites have various pharmaceutical roles, mainly antimicrobial, antioxidant, antiviral, antitumor, anti-inflammatory, antigenotoxic, antiherbivore, analgesic, enzyme inhibitory and antipyretic characteristics [2-8].
Natural bioactive products such as substances from lichen have an important potential for antimicrobial treatment. Moreover, these natural substances have been used for medicinal drug development in pharmaceutical industries.
There are many antimicrobial activity studies about crude lichen extracts but there are a few studies on their pure substances while they have strong antimicrobial activity. One of them is thamnolic acid found in some lichens Lepraria spp. [9,10]. Usnea florida (present study), Thamnolia vermicularis [11] and Cladonia incrassata [12].
Therefore, the assessment of the antimicrobial activities of the methanol, acetone and chloroform extracts of the lichens Usnea florida and its thamnolic acid constituent as a secondary metabolite is presented in the current study.
Materials and Methods
Lichen samples
The collection of Usena florida (L.) Weber ex F.H. Wigg was performed in Abant Mountains, Bolu Province, Turkey at 1080 m on the 19th of August 2015. The sample for demonstration purposes is deposited at the Herbarium of Anadolu University, Department of Biology (ANES). Standard keys were used for the identification of the lichens examined [13,14].
Preparation of the lichen extracts
100 ml of methanol, chloroform, and acetone were used in order to acquire extracts obtained from finely ground dry thalli of the lichen (10 g) in a Soxchlet extractor. The filtration of the extracts was performed and following this they were concentrated under decreased pressure in a rotary evaporator and stored at the temperature of -18°C until they were used in the experiments.
Microorganisms
In the present study, the following bacteria, yeasts, and filamentous fungi were used as experimental organisms: Bacillus cereus ATCC 10876, Bacillus subtilis NRRL NRS-744, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 51299, Enterobacter aerogenes NRRL B-3567, Klebsiella pneumoniae ATCC 700603, Listeria monocytogenes ATCC 19111, Micrococcus luteus NRRL B-4375, Mycobacterium tuberculosis H37Rv (ATCC 27294), Pseudomonas aeruginosa ATCC 27853, Proteus vulgaris NRRL B-123, Staphylococcus aureus ATCC 6538, Salmonella typhimurium ATCC 14028, and Yersinia enterocolitica Y53 bacteria, Candida parapsilosis ATCC 22019, Candida albicans ATCC 90028, Candida globrata ATCC 90030, and Candida krusei ATCC 6258 yeasts, and Aspergillus niger ATCC 9807, Aspergillus flavus ATCC 9807, Aspergillus parasiticus NRRL 465, Fusarium moniliforme NRRL 2374, Aspergillus fumigatus NRRL 113, Rhizopus sp., Alternaria brassicola, Sclerotium rolfsii, and Fusarium solani filamentous fungi obtained from our laboratory.
Antimicrobial activity of chloroform, methanol and acetone extracts to test microorganisms
Mueller-Hinton agar plates were used to maintain bacterial cultures. Potato dextrose (PD) agar and Sabouraud dextrose (SD) agar were used for the maintenance of fungal cultures. Test bacteria were obtained from bacterial cultures the incubation of which was incubated for 24 h at 37°C on Mueller-Hinton agar substrate and the dilution of which was prepared in accordance with the 0.5 McFarland standards to about 108 CFU/ml. Fresh mature cultures at the age of 3 to 7 days growing at 30°C on a PD agar substrate were used for the preparation of fungal spore suspensions. Sterile 0.1% Tween 80 was used for the rinsing of the spores and their further dilution to about 106 CFU/ml was performed in accordance with the procedural recommendations of CLSI [15]. The lichen extracts, 125 mg acetone, 215 mg methanol, and 75 mg chloroform were dissolved in 1 ml of the same solvent including 60 blank sterile antibiotic disks (7 mm diameter) for the disk diffusion test. The solvents were evaporated in a vacuum oven. As negative control agents on the plates, treated and dried disks of pure chloroform, methanol and acetone were used. The final concentration of one disk was 2.08 mg for acetone, 3.58 mg for methanol, and 1.25 mg for chloroform. The measurement of the inhibition zone of the extract’s specific concentration was performed using Kirby and Bauer disk diffusion method in order to investigate the microorganisms’ sensitivity to methanol, acetone and chloroform extracts of the lichen [16-18]. Mueller-Hinton agar, SD agar, and PD agar were seeded with the appropriate inoculum for bacteria, yeast, and filamentous fungi, respectively. The disks with the diameter of 7 mm containing lichen extracts were placed on the test plates. The diameter of the inhibition zone around the disk was measured in order to identify antimicrobial activity. Ketoconazole (for fungi) and streptomycin (for bacteria) were utilized as controls. All experiments were repeated three times.
Determination of the MICs of the extracts
The determination of the minimal inhibitory concentration (MIC) was performed using the broth tube dilution method. The starting solutions of extracts were prepared using a 10 mg/ml concentration dissolved in 20% DMSO. Mueller-Hinton broth was used for the preparation of the extracts’ two-fold dilutions for bacterial cultures and SD broth was used for fungal cultures in experimental tubes. A number of dilutions, the concentrations of which varied between 10 mg/ml and 19.53 μg/ml, were utilized in the test for every extract against only sensitive microorganisms. The visible growth of the microorganisms was provided and thus the minimal inhibitory concentration was identified. The minimal inhibitory concentration (MIC) for the microorganism examined at the determined lichen extract concentration was described as the boundary dilution with no visible growth. To control the growth inhibition, streptomycin was utilized as a positive control for bacteria and ketoconazole was utilized as a positive control for fungi. All tests were repeated three times.
Bioautographic method with thin layer chromatography
Following a preparatory thin layer chromatography (TLC) study, it was determined that the methanol extract was the solution with the highest concentration among the extracts. The methanol extract of Usnea florida of the specific volume was obtained and placed on silica gel TLC plates (Merck, Silica gel 60 F254). Following this, the development of the TLC plates was performed in three solvent systems which are generally used in the TLC of lichen substances. A mix of toluene/ dioxane/glacial acetic acid (36:9:1 v/v/v) was present in solvent system A, hexane/diethyl ether/formic acid (24:8:4 v/v/v) were present in solvent system B, and there were toluene/glacial acetic acid (20:3 v/v) in solvent system C [19]. Afterwards, the plates that had been developed were placed into petri dishes with the cover of thin nutrient agar. In conclusion, soft nutrient agar containing experimental microorganisms (108 CFU/ml) was spread in 2 mm thickness to the petri dishes and their incubation was performed for 24-48 h at the temperature of 35°C. Antimicrobial activity was demonstrated by two substances on the TLC plates and afterwards these substances were determined to be usnic acid and thamnolic acid.
Checking the Rf values of the substances in various solvent systems with the values in the literature, as well as their melting points and IR spectra, constitutes the basis for their characterization [20-24]. Since the study of usnic acid was performed in our previous work [25], we examined the effect of thamnolic acid content. A polarimeter was used for the identification of the thamnolic acid’s enantiomeric form. This pure thamnolic acid was utilized in order to identify its antimicrobial activity toward all of the test microorganisms.
Minimal inhibitory concentration (MIC) of thamnolic acid
The broth microdilution method with 96-well microtiter plates was employed for the determination of the minimal inhibitory concentration (MIC) of thamnolic acid. A number of dilutions, the concentrations of which varied between 25.6 mg/ml and 50 μg/ml, was utilized for each test microorganism. Thamnolic acid was dissolved in DMSO (20%) and thus its starting solution was acquired. Mueller-Hinton broth was used for the preparation of two-fold dilutions in the case of bacterial cultures and SD broth was utilized in the case of fungal cultures. The determination of the minimal inhibitory concentration was performed using resazurin, which indicates oxidation-reduction and is employed for the assessment of microbial growth. Resazurin represents a non-fluorescent dye of blue color which turns pink and fluorescent in the case of reduction to resazurin with oxidoreductases inside viable cells. The minimal inhibitory concentration (MIC) for the microorganism examined at the determined concentration was described as the boundary dilution with no altering color of resazurin [26]. To control the growth inhibition, streptomycin was utilized as a positive control for bacteria and ketoconazole was utilized as a positive control for fungi. At the same time, as a negative control for the solvents’ impact, a DMSO solution was utilized. All tests were repeated three times.
Microplate alamar blue assay (MABA) for antitubercular activity of the lichen extracts and pure thamnolic acid
Mycobacterium tuberculosis H37Rv (ATCC 27294) was provided from American Type Culture Collection (ATCC) cell bank. The cells were cultivated in ATCC® Medium 1395: Middlebrook 7H9 broth with ADC enrichment at 37°C for 30 days. The adjustment of the cultures’ turbidity to McFarland standard number 1 was performed. 5-0.0097 mg/ml were prepared for lichen extracts and rifampicin (Sigma, R3501, China) and 2-0.0039 mg/ml for thamnolic acid. The incubation of all black, clear-bottomed, 96-well plates (Corning 3340, USA) was performed at the temperature of 37°C for the period of 7 days. A newly made 1:1 mixture containing Alamar Blue reagent (1:10 dilution, Invitrogen, 1025, USA) and 10% Tween 80 was put to one well in a group of the positive controls on the 7th day of the incubation. The further incubation of the plates was performed at the temperature of 37°C for the 24 h period. The reagent mixture was put in each well of the micro plate in case the well’s content became pink [27].
Results
Table 1 contains the data on the antimicrobial activity of the lichen Usnea florida extracts and MIC values toward the microorganisms examined.
| Usnea florida extracts | Thamnolic acid (μg/ml) | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test microorganisms | Methanol | Chloroform | Acetone | Streptomycin | Ketoconazole | ||||||
| D | MIC (μg/ml) | D | MIC (μg/ml) | D | MIC (μg/ml) | MIC (μg/ml) | D | MIC (μg/ml) | D | MIC (μg/ml) | |
| Bacteria | |||||||||||
| B. cereus | 24 | 78.12 | 20 | 78.12 | 23 | 78.12 | 400 | 30 | 4.88 | ||
| B. subtilis | 25 | 156.2 | 22 | 156.2 | 24 | 78.12 | 400 | 28 | 4.88 | ||
| E. coli | - | - | - | - | |||||||
| E. aerogenes | 15 | 312.5 | 20 | 312.5 | 16 | 156.2 | - | 26 | 9.76 | ||
| E. faecalis | 15 | 156.2 | 15 | 156.2 | 18 | 156.2 | - | 30 | 9.76 | ||
| K. pneumoniae | - | - | - | - | |||||||
| L. monocytogenes | 15 | 156.2 | 15 | 312.5 | 15 | 156.2 | 200 | 28 | 39.06 | ||
| M. luteus | 19 | 78.12 | 20 | 312.5 | 15 | 78.12 | 200 | 22 | 19.53 | ||
| P. vulgaris | 18 | 156.2 | 15 | 312.5 | 16 | 156.2 | 400 | 20 | 19.53 | ||
| P. aeruginosa | - | - | - | - | |||||||
| S. aureus | - | - | - | - | |||||||
| S. typhimurium | - | - | - | - | |||||||
| Y. enterocolitica | 15 | 156.2 | 15 | 156.2 | 14 | 156.2 | - | 20 | 39.06 | ||
| Yeasts | |||||||||||
| C. albians | - | - | - | - | |||||||
| C. krusei | 13 | 625 | 15 | 625 | 14 | 156.2 | 400 | 35 | 19.53 | ||
| C. paropilopsis | 15 | 312.5 | 14 | 625 | 15 | 156.2 | - | 40 | 9.76 | ||
| C. globrata | 14 | 625 | 15 | 625 | 15 | 312.5 | - | 38 | 9.76 | ||
| Filamentous fungi | |||||||||||
| F. solani | - | - | - | - | |||||||
| F. moniliforme | - | - | - | - | |||||||
| A. alternata | - | - | - | 400 | 32 | 39.06 | |||||
| A. niger | - | - | - | - | |||||||
| A. flavus | - | - | - | - | |||||||
| A. parasiticus | - | - | - | - | |||||||
| A. fumigatus | - | - | - | 400 | 35 | 78.12 | |||||
| S. rolfsii | - | - | - | 200 | 38 | 19.53 | |||||
| D: Diameter; -: No activity. | |||||||||||
Table 1: Antimicrobial activity of methanol, chloroform and acetone extracts of Usnea florida and their Minimum Inhibitory Concentration (MIC).
The methanol, acetone and chloroform extracts of the lichen Usnea florida showed much the same effect to tested microorganisms. Antibacterial activity was determined in all extracts to Gram-positive bacteria Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, Micrococcus luteus, Listeria monocytogenes, and Gram-negative bacteria Enterobacter aerogenes, Proteus vulgaris, Yersinia enterocolitica. However, the lichen extracts examined were not sensitive to Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and Salmonella typhimurium.
The greatest sensitivity to the species examined was demonstrated by the species Bacillus cereus and Bacillus subtilis with the inhibition zone of 20 mm or larger. The extracts of the lichen Usnea florida also showed an activity to yeasts except for Candida albicans species. An antifungal activity was not determined for all of the filamentous fungi.
The largest inhibition zone (25 mm) was measured in the methanol extract relative to the species Bacillus subtilis. The extracts of this lichen showed a weaker activity to Candida species than bacteria. The inhibition zone for Candida species ranged between 13-15 mm. The inhibition zones in relation to the bacteria were large. They were within the range of 23-24 mm for the acetone extract and 24-25 mm for the methanol extract. The MIC values of the extracts were found to be between 78.12-312.50 μg/ml for bacteria and 156.20-625 μg/ml for yeasts (Table 1). After the antibacterial activity had been determined and the MIC values of the extracts of Usnea florida had been calculated, the determination of the substances demonstrating antibacterial activity was performed with the bioautographic method.
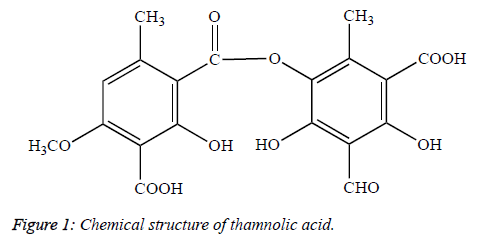
The activity of two substances in the extracts, usnic acid and thamnolic acid, was determined. The place of the spot, which demonstrated the inhibition zone, varied on every TLC plate in accordance with the TLC development solvent system. The isolation of one minor substance was performed with the preparative TLC, its characterization was performed with the Rf values and melting point, and as a result, it was determined to be thamnolic acid (C19H16O11, MA=420.32) (Figure 1).
The activities of thamnolic acid against twelve bacteria, four yeasts, and seven filamentous fungi, the identical microorganisms which were utilized in the research on the extract, were examined and Table 1 represents their MIC values obtained. Although the extracts did not demonstrate antimicrobial activity to the filamentous fungi examined, pure thamnolic acid showed the antifungal effect against Alternaria alternata and Sclerotium rolfsii.
Likewise, Enterobacter aerogenes, Enterococcus faecalis, and Yersinia enterocolitica bacteria were not sensitive to the extracts while being sensitive to pure thamnolic acid. Most probably, the amount of thamnolic acid was lower than its MIC in the extracts.
In vitro antimycobacterial effects of the lichen extract and thamnolic acid were also investigated against the drug resistant M. tuberculosis H37Rv strain with the Microplate Alamar Blue Assay (MABA). Lichen extracts and thamnolic acid exhibited antimycobacterial activity with a MIC value of 78.12 μg/ml and 250 μg/ml, respectively.
Discussion
For the first time, the study was conducted on the antimicrobial and especially antituberculosis activity of the lichen Usnea florida and thamnolic acid constituent. In accordance with the findings acquired in this research, no differences in the antimicrobial activity were determined between the extracts by the type of the extracting solvent. In the studies carried out by other researchers on the antimicrobial activity of lichen extracts, similar aspects as well as differences were identified compared to the findings of our study [7,28-34]. The fact that there are various antimicrobial active components is the possible reason for the above mentioned similar aspects and differences in the antimicrobial activity of the extracts of various lichen species. The findings of the present study demonstrate that the extracts examined showed a strong and same antibacterial activity relative to the antifungal activity. As a result of the study conducted by Hugo and Russell, a higher sensitivity of bacteria to antibiotics in comparison with fungi was determined [35]. This can be caused by the differences that are present in the cell wall of bacteria and fungi [36-38]. According to the results, a significant antituberculosis activity was also determined in lichen extracts and thamnolic acid. The findings of our study showed that a significant antibacterial impact was demonstrated by lichen extracts. Therefore, lichens can contribute to the treatment of various diseases, the cause of which is the microorganisms mentioned above.
Acknowledgements
The financial support for this study was provided by Anadolu University, Research Project 1605F401.
References
- Rankovic B, Kosanic M. Lichens as a Potential Source of Bioactive Secondary Metabolites. Springer Int Publ 2015; 1-26.
- Lawrey JD. Biological role of lichen substances. Bryologist 1986; 89: 111-122.
- Ingolfsdottir K, Hjalmarsdottir MA, Sigurdsson A, Gudjonsdottir GA, Brynjolfs-dottir A, Steingrimsson O. In vitro susceptibility of Helicobacter pylori to protolichesterinic acid from the lichen Cetraria islandica. Antimicrob. Agents Chemother 1997; 41: 215-217.
- Huneck S. The significance of lichens and their metabolites. Naturwissenschaften 1999; 86: 559-570.
- Ingofsdottir K. Usnic acid. Phytochemistry 2002; 61: 729-736.
- Haraldsdottir S, Gudlaugsdottir E, Ingolfsdottir K, Ogmundsdottir HM. Anti-proliferative effects of lichen-derived lipoxygenase inhibitors on twelve human cancer cell lines of different tissue origin in vitro. Planta Med 2004; 70: 1098-1100.
- Kosanic M, Rankovic B. Antibacterial and antifungal activity of different lichens extracts and lichen acid. Res J Biotechnol 2011; 6: 23-26.
- Fernandez-Moriano C, Gomez-Serranillos MP, Crespo A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm Biol.2016; 54: 1-17.
- Orange A, Wolseley P. Two new thamnolic acid-containing Lepraria species from Thailand. Lichenologist 2005; 37: 247-250.
- Elix JA, Spielmann AA, Ovstedal DO. A new Lepraria from Brazil. Hoehnea 2010; 37: 39-41.
- Guo J, Li ZL, Wang AL, Liu XQ, Wang J, Guo X, Jing YK, Hua HM. Three new phenolic compounds from the lichen Thamnolia vermicularis and their antiproliferative effects in prostate cancer cells. Planta Med 2011; 77: 2042-2046.
- Dieu A, Millot M, Champavier Y, Mambu L, Chaleix V, Sol V, Gloaguen V. Uncommon chlorinated xanthone and other antibacterial compounds from the lichen Cladonia incrassate. Planta Med 2014; 80: 931-935.
- Randlane T, Tõrra T, Saag A, Saag L. Key to European Usnea species. Bibliotheca Lichenologica 2009; 100: 419-462.
- Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA. The lichens of Great Britain and Ireland. British Lichen Soc London (2nd edn.) 2009.
- CLSI (Clinical and Laboratory Standards Institute). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved Standard. CLSI document M38-A2. Clin Lab Stand Inst (2nd edn.) 2008.
- CLSI (Clinical and Laboratory Standards Institute). Performance standards for antimicrobial disk susceptibility tests; approved standard. CLSI document M02-A11. Clin Lab Stand Inst (11th edn.) 2012.
- Iftikhar T, Zia A. Niaz M, Ashraf I, Abbas SQ, Lee KJ, Haq IU. Mutation induced enhanced biosynthesis of lipases by Rhizopus oligosporus var. microsporus. Pak J Bot 2010; 42: 1235-1249.
- Jabeen R, Ashraf M, Ahmad I, Iftikhar T. Purification and bioassays of bioactive fraction from Curcuma longa against Xanthomonas oryzae pv. oryzae causing blb disease in rice. Pak J Bot 2011; 43: 1335-1342.
- Culberson CF, Amman K. Standardmethode zur Dünnschichtchromatographie von Flechtensubstanzen. Herzogia 1979; 5: 1-24.
- Huneck S, Yoshimura I. Identification of Lichen Substances. Spring Berlin 1996; 11-123.
- Culberson CF, Culberson WL, Johnson A. Second supplement to chemical and botanical guide to lichen products. The American Bryological and Lichenological Society, St. Louis, Missouri 1977.
- Orange A, James PW, White FJ. Microchemical Methods for the Identification of Lichens. British Lichen Society, London 2001.
- Schumm F. Dünnschichtchromatogramme auchfür den Amateur moglich. Aktuelle Lichenologische Mitteilungen, NF 9 Essen 2002; 8-22.
- Edwards HGM, Newton EM, Wynn-Williams DD. Molecular structural studies of lichen substances II: atranorin, gyrophoric acid, fumarprotocetraric acid, rhizocarpic acid, calycin, pulvinic acid dilactone and usnic acid. J Mol Struct 2003; 651-653: 27-37.
- Yilmaz M, Türk AÖ, Tay T, Kivanc M. The antimicrobial activity of extracts of the lichen Cladonia foliacea and Its (-)-usnic acid, atranorin, and fumarprotocetraric acid constituents. Z Naturforsch. 2004; 59: 249-254.
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals methods. Methods 2007; 42: 321-324.
- Kaplancikli ZA, Altintop MD, Sever B, Cantürk Z, Özdemir A. Synthesis and in vitro evaluation of new thiosemicarbazone derivatives as potential antimicrobial agents. Hindawi Publishing Corporation J Chem 2016; 7.
- Madamombe IT, Afolajan AJ. Evaluation of antimicrobial activity of extracts from South African Usnea barbata. Pharmaceut Biol 2003; 41: 199-202.
- Candan M, Yilmaz M, Tay T, Kivanç M, Türk H. Antimicrobial activity of extracts of the lichen Xanthoparmelia pokornyi and its gyrophoric and stenosporic acid constituents. Z Naturforsch C 2006; 61: 319-323.
- Candan M, Yilmaz M, Tay T, Erdem M, Türk AO. Antimicrobial activity of extracts of the lichen Parmelia sulcata and its salazinic acid constituent. Z Naturforsch C 2007; 62: 619-621.
- Rankovic B, Mišic M, Sukdolak S. Antimicrobial activity of the lichens Cladonia furcata, Parmelia caperata, Parmelia pertusa, Hypogymnia physodes and Umbilicaria polyphylla. British J Biomed Sci 2007; 64: 143-148.
- Rankovic B, Mišic M, Sukdolak S. The antimicrobial activity of substances derived from the lichens Physcia aipolia, Umbilicaria polyphylla, Parmelia caperata and Hypogymnia physodes. World J Microbiol Biotechnol 2008; 24: 1239-1242.
- Rankovic B, Rankovic D, Kosanic M, Maric D. Antioxidant and antimicrobial properties of the lichen Anaptychya ciliaris, Nephroma parile, Ochrolechia tartarea and Parmelia centrifuga. Cent Eur J Biol 2010; 5: 649-655.
- Rankovic B, Kosanic M. Activities of different extracts of Lecanora atra, Lecanora muralis, Parmelia saxatilis, Parmelia sulcata and Parmeliopsis ambigua. Pak J Bot 2012; 44: 429-433.
- Hugo WB, Russell AD. Pharmaceutical microbiology London Blackwell Sci Publ (3rd edn.) 1983; 1-351.
- Yang Y, Anderson EJ. Antimicrobial activity of a porcine myeloperozidase against plant pathogenic bacteria and fungi. J Appl Microbiol 1999; 86: 211-220.
- Ruiz-Herrera J. Chemical composition of the fungal cell. London CRC Press 1992; 5-40.
- Griffin DH. Molecular architecture. Wiley Liss New York (2nd edn.) 1994; 65-74.