- Biomedical Research (2012) Volume 23, Issue 2
Screening for aminoacidurias and organic acidurias in patients with metabolic or neurological manifestations
Vidya S. Patil*, Rama Jailkhani, Dhiraj J. Trivedi, Shreerang.P. Kulkarni, Aparna A. Sagare, Rakesh Mudaraddi, Anil Bargale
Department of Biochemistry, SDM College of Medical Sciences and Hospital, Dharwad-580009, Karnataka, India.
- *Corresponding Author:
- Vidya.S.Patil
Department ofBiochemistry
SDM College of Medical Sciences and Hospital
Dharwad 580009
Karnataka
India.
Accepted date: January 17 2012
Abstract
Inborn errors of metabolism (IEM) are a group of inherited disorders occurring due to a single gene defect, resulting in accumulation of an abnormal metabolite leading to varied manifestations and complications. Early recognition and treatment are the best determinants of outcome in such patients. Objective: With the objective of providing a guide towards early diagnosis of IEM among patients having strong clinical suspicion, we have screened 128 urine samples from patients with either metabolic or neurological features. Method: Urine samples were analysed for abnormal constituents like reducing sugar, proteins, ketone bodies by routine laboratory chemical tests. Special tests were done for phenylketones, organic acids, ketoacids, tyrosine, mucopolysachharides supported by thin layer chromatography for aminoacidurias. Results: Most common positive tests reported in our study are, non specific generalized aminoacidurias (58%), branched chain aminoacidurias (14%), tyrosinuria (13%), methlymalonic aciduria (7%) followed by mucopolysaccharidosis (4%) and phenylketonuria (2%). The common clinical manifestations observed among all participants were neurological features like convulsions (25.7%), delayed milestones (17.9%), and followed by metabolic acidosis (17.2%) and hypoglycemia (10.1%). Conclusion: High index of suspicion from clinicians supported by preliminary screening tests can aid in early presumptive diagnosis, which helps in initiating early treatment to prevent lethal neurological complications.
Keywords
Aminoacidurias; Organic acidurias; TLC for aminoacids; Urine screening for IEM.
Introduction
Inborn errors of metabolism (IEM) comprise a diverse group of heterogeneous disorders manifesting in pediatric population. The metabolic error is caused due to lack or deficiency of key enzyme or coenzyme of an intermediary metabolic pathway causing urea cycle defects, aminoacidopathies, organic acidemias, fatty acid oxidation defects and errors in energy metabolism [1].
The most common clinical presentation associated with IEM includes CNS manifestations, lethargy, poor feeding, recurrent vomiting and failure to thrive. Acute encephalopathy due to hyperammonemia, is especially observed in urea cycle defects and many organic acidemias. Among the inborn errors, the largest group typically associated with overwhelming metabolic acidosis in infancy is the group of organic acidemias, like methylmalonic acidemia, propionic acidemia and isovaleric acidemia. Glycogen storage disorders may be associated with hepatomegaly and hypoglycemia. Mucopolysaccharidoses typically manifest at later stages of life with coarse facial features, hepatospelnomegaly, skeletal abnormalities and hernias [2].
Earlier studies carried out by Kumta et al, reported that 5.75% cases of mental retardation were due to metabolic disorders [3]. Screening of 112269 newborn babies for aminoacid disorders by Verma et al reported that tyrosinemia, Maple syrup urine disease (MSUD), Phenylketonuria (PKU), hyperglycinemia, homocystinuria and alkaptonuria were among the major aminoacidopathies. Amongst the storage disorders, commonly reported were mucopolysaccharidoses, glycogen storage disorder and galactosemia [4]. Kamate et al attributed the high incidence of IEM (2.6%) among patients admitted to NICU to the high rate of consanguinity found in their study population [5].
Most IEMs present in a non specific manner. Hence, it is indeed essential to screen the newborns for IEM, especially those presenting with metabolic or neurological disorders, so that treatment wherever available, can be initiated at the earliest to reduce morbidity and mortality rates. Hence, we intended to carryout basic urine screening of newborns with high clinical suspicion for IEM.
Objective
The objective of the present study was to carry out preliminary screening on urine samples from pediatric population with either metabolic or neurological manifestations for inborn errors of metabolism.
Material and Methods
The present study is a cross sectional time bound study carried out from 2007 to 2009. A total of 128 samples were analysed from suspected cases of IEM. Screening tests were considered in patients who presented with any of the following features like convulsions, failure to thrive, regression of milestones, recurrent vomiting, mental retardation, skeletal deformities, organomegaly, metabolic acidosis, hypoglycemia or jaundice. In many of the cases family history and sibling history were much contributory for diagnosis, like history of consanguinity, sibling deaths due to similar illness, repeated abortions etc. 25 ml of random sample of urine was collected in a sterile plastic container with 5 drops of 6N HCl as preservative. Sample was centrifuged at 5000 rpm for 15 minutes and supernatant was analysed for all physical and chemical parameters. Following laboratory investigations were included in the screening protocol [6]- Benedicts test for reducing substances, Suphosalycilic acid test for proteins, Rotheras test for Ketone bodies, Ferric chloride test for Phenylketonuria, Dintrophenylhydrazine test for alpha keto acids, Nitrosonaphthol test for tyrosine, Para nitroaniline test for methylmalonic aciduria, Cyanide nitroprusside test for cysteine and homocysyteine, Ammoniacal silver nitrate test for homocysteine, Methylene blue spot test and Cetyl pyridinium chloride (CPC) citrate turbidity test for mucoplysaccharidoses, Test for porphobilinogen, Thin layer chromatography for aminoacids and carbohydrates [7].
Additional investigations which supported our diagnosis were Arterial blood gas analysis, Liver function tests, Ammonia, Lactic acid, Serum electrolytes, Urea and creatinine. Any positive cases reported by these investigations were referred to higher centers for confirmation.
Results
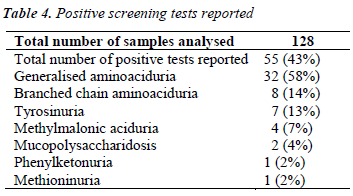
During the course of our study, we have analysed 128 samples, among which 55 were given presumptive diagnosis of IEM based on positive screening tests, urinary aminoacidogram by TLC and clinical correlation.
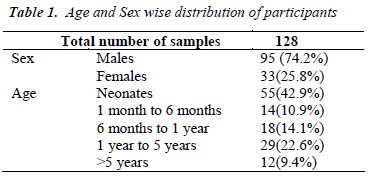
Table-1 shows the age and sexwise distribution of suspected IEM cases. There was preponderance of males (74.2%) compared to females (25.8%) in the study group. Age distribution showed that maximum participants were neonates (42.9%) and only 9.4% cases were >5 years of age, indicating that late onset disorders are less prevalent compared to acute conditions manifesting in newborns.
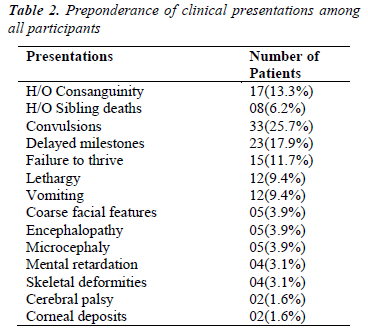
Analysis of various clinical presentations and available family history is shown in Table-2. The data suggests that the most common presentations were convulsions (25.7%), delayed milestones (17.9%), and failure to thrive (11.7%). Common laboratory features were metabolic acidosis (17.2%) followed by hypoglycemia (10.1%) (Table- 3).
Table 4 shows the abnormal cases reported among a total of 128 cases analysed.
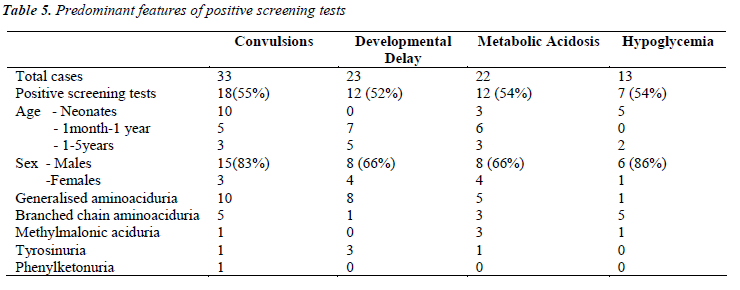
Further detailed analysis of the data (Table-5) showed that, among the children who presented with convulsions, 55% showed positive screening tests. Maximum were in the neonatal age group. Out of these 18 positive cases, 10 were generalized aminoacidurias, 5 were branched chain aminoaciduria, and one cases each of methylmalonic aciduria, tyrosinuria and phenylketonuria.
Among children who presented with developmental delay, >50% showed positive screening tests. Most of them were between 6 months to 3 years. Among these 12 cases, 8 were having generalized aminoaciduria, 3 presented with tyrosinuria and 1 showed branched chain aminoaciduria.
Discussion
Most of the IEM usually present with CNS symptoms. Seizures were a dominant symptom (26%) followed by delayed milestones (18%) as is reported in literature [8]. Various studies have reported that, over one-third of the IEMs are characterized by CNS involvement and neurological symptoms are the most prominent clinical problems associated with them. Routine screening for IEM in children with developmental delay has a diagnostic yield of approximately 1% that can increase to 5% in specific situations such as in the case of relatively homogenous and isolated populations or if there are clinical indicators [9].
Metabolic acidosis was the second common finding among our study subjects (17%). Among them >50% were positive for screening tests. Three of them were positively diagnosed as cases of methlymalonic aciduria, 3 had branched chain aminoaciduria and one showed tyrosinuria. An increased anion gap (>25mmol/L) observed in 2 cases was an interesting finding, which may be due to accumulation of fixed acids and/or organic acids. The group of organic acidemias including methlymalonic, propionic and isovaleric acidemias are among the important causes for wide anion gap metabolic acidosis [9].
Generalised aminoaciduria
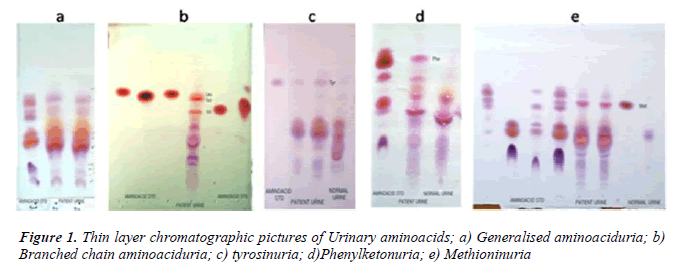
In our study, maximum cases (58%) were nonspecific generalized aminoacidurias. The predominant aminoacids found in urine were glycine, serine, alanine, glutamate using TLC (Fig 1a). Our findings can be attributed to secondary abnormalities of aminoacid concentration in plasma and urine like, severe hepatocellular disease, renal tubular disease, catabolic states, malnutrition, malignancy, infections, pregnancy, vitamin deficiencies, burns and other injuries. These secondary causes have to be ruled out before attributing it to a specific inborn metabolic disorder [9]. In a 30 year survey, Mattingley JM reported that nonspecific generalized aminoaciduria was the most frequent abnormality found, comprising 70% of abnormal results, with cystine-lysinuria to be the next most common disorder [10].
Branched chain aminoaciduria
During the course of our study, we found 8 cases with branched chain aminoaciduria. Most commonly the patients had wide anion gap metabolic acidosis and ketonuria. DNPH test for alpha keto acids was positive in 5 patients. TLC showed significant excretion of branched chain aminoacids (Fig 1b). Classic Maple Syrup Urine Disease (MSUD) has a neonatal onset with encephalopathy, and is the most severe and common form of MSUD [11]. Few of these patients may be responsive to vitamin thiamine [3]. Dietary therapy has to be initiated within 2 weeks of birth to achieve normal intellect and it should be a long term mode of treatment [12].
Tyrosinuria
We are reporting here 7 cases of transient tyrosinuria who were in the age group of 3 months to 3 years and all of them were males. They manifested with developmental delay, failure to thrive and convulsions. History of consanguinity was present in three of them. Urine gave strong positive nitrosonaphthol test for the presence of tyrosine, which was confirmed by the presence of tyrosine in urinary TLC (Fig 1c).
Transient neonatal tyrosinemia is considered to be a benign condition, although there is lack of recent studies to confirm this information. In older studies, a reduction in motor activity, developmental alterations, lethargy and intellectual defect in children was assessed at 4-5years of age. Oliviera et al have reported highest positivity for tyrosinuria (29%) giving nitrosonaphthol test positive which they attributed to metabolic immaturity. This condition is believed to be the most common alteration in the metabolism of aminoacids in human beings, with an incidence of 10% having been described among full term newborns and 30-50% among premature newborns [1]. Neonatal tyrosinemia is associated with increased excretion of tyrosine and its metabolites in 0.2 to 10% of neonates [13]. This condition does not have an exclusive genetic determinant (late maturation of hydroxyphenylpyruvate dehydrogenase and tyrosine aminotransferase), but is also related to prematurity, high protein ingestion during the first days of life (>3g of protein/Kg), low birth weight, low ingestion of vitamin C, among other factors [1].
In a child with tyrosinuria and high plasma tyrosine levels, Tyrosinemia type I has to be ruled out especially if the child is presenting with acute hepatic crisis, hepatomegaly or bleeding diathesis precipitated by intercurrent illness. In such a case, confirmation is done by increased plasma and urine succinylacetone levels and deficiency of the enzyme fumaryl acetoacetate hydroxylase [14].
Methylmalonic aciduria
During the period of our study, we have reported 4 cases of methylmalonic aciduria, which gave strong positive pnitroaniline test. One of the patient had characteristic severe metabolic acidosis with wide anion gap of >25mmol/L, urine gave positive for ketone bodies and the child had severe hypoglycemia (RBS- 47mg%). All the above features are suggestive of organic acidemias. The presence of ketone bodies in urine rules out fattyacyl COA dehydrogenase deficiency [9]. Para-nitroaniline test for methylmalonic acid is strongly positive. Metabolic ketoacidosis is a clinical hallmark of methylmalonic aciduria in infants. A study from china shows methylmalonic aciduria was the common IEM found and B12 deficiency was the commonest cause [15]. Therapy consists of protein restriction, restriction of methylmalonate precursors and pharmacologic doses of vitamin B12 [16].
Mucopolysaccharidosis
We came across 20 cases with positive CPC citrate turbidity test for mucopolysaccharidosis, but only 10 cases among that gave methylene blue spot test positive. Among these only two cases were confirmed based on skeletal deformities. This indicated that CPC test has more tendency to give false positive results, as also reported by Oliviera et al, and may be usually associated with either reducing substances, proteins or altered aminoacid pattern especially generalized aminoaciduria [1]. Hence methylene blue spot test was performed, where 10 cases gave positive results. These tests were supported by strong clinical presentations in the form of skeletal abnormalities in our patient like pigeon chest, enlargement of wrist joint, horizontal ribs which were spatula shaped on X-ray, short bullet shaped phalanges, thick skull bone and corneal opacity.
Phenylketonuria (PKU)
PKU is and autosomal recessive disorder most commonly caused by a mutation in the gene coding for phenyalanine hydroxylase. In our patient with PKU, there was microcephaly, muscular hypotonia, cerebral palsy with hypopigment hairs which are usually associated with classical condition [16]. Classically, ferric chloride test for phenylketones was positive and urine TLC showed increased excretion of phenylalanine (Fig 1d). The neurological complications could be due to delay in initiation of prompt dietary therapy as it was not screened for IEM. It could have been prevented if the condition was diagnosed at birth and dietary restriction of phenylalanine was initiated.
Methioninuria
We have reported one case of significant methioninuria which was associated with generalised aminoaciduria (Fig 1e). This finding suggests one of these important metabolic defects – cystathionine beta synthase deficiency or hepatic methionine adenosyl transferase deficiency. Studies carried out to identify severe hypermethioninemia have reported that it was related to ingestion of infant protein hydrolysate formula with high methionine content. Extreme hypermethioninemia resulted in cerebral edema in few of these patients [17]. In the absence of homocysteine and cystathionine in urine as found in our case, it could be suggested that an alternate pathway of methionine breakdown exists in man, and that an enzymatic block possibly in oxidative decarboxylation of the alpha keto acid of methionine could have accounted for the presentation. Such a case was reported to be associated with generalised aminoaciduria, cirrhosis, islet cell hyperplasia and renal tubular degeneration [18].
Conclusion
Screening for IEM in suspected cases should always be done at an earlier age. Positive screening tests give presumptive diagnosis of IEM. Early recognition of IEM by screening tests in combination with strong clinical suspicion will help the clinicians to initiate prompt early treatment to prevent lethal neurological complications and developmental delay. These simple screening tests are highly cost effective and will reduce the economic burden of the patients. Definitive diagnosis by specified tests can be advised for those patients who are positive on screening tests. This will help to overcome the economic constraints in diagnosis of IEM. Hence, there is a need to provide simple screening tests for IEM, as these disorders are not uncommon among those children presenting with non specific symptoms.
Acknowledgements
We are thankful to M.D and Principal for giving us support in carrying out the study. We also thank our pediatrics department, local pediatricians Dr.L.H.Bidari, Dr.Rajendra Salagare, Dr.Mahesh Kore, KIMS hospital Hubli for sending us the samples. We thank Mr.Kumar C.A, Mr.Guruswamy and Mr.Ajay Kotkar for providing us technical support.
References
- Oliveira AC, Nunes AM, Santos D, Martins AM, D’Almeida V. Screening for Inborn errors of metabolism among newborns with metabolic disturbance and/or neurological manifestations without determined cause, Sao Paulo Med J 2001; 119 (5): 160-164
- Burton BK. Inborn errors of metabolism in Infancy: A guide to Diagnosis, Pediatrics 1998; 102 (6): e69.
- Kumta NB. Inborn errors of metabolism (IEM)-an Indian perspective. Indian J Paediatr 2005; 72: 325-332.
- Verma IC. Burden of genetic diseases in India. Indian J Pediatr 2000; 67:893-898.
- Kamate M, Chetal V, Kulgod V, Patil V, Christopher R. Profile of Inborn errors of metabolism in a tertiary care centre PICU. Indian J Pediatr 2010; 77 (1):57-60.
- Christenson RH, Azzay HME, ‘Amino acids’ In: Burtis CA and Ashwood ER (Eds.) Teitz Textbook of Clinical Chemistry, 3rd edition, Philadelphia, W.B Saunders, 1999; 444-476.
- Ersser RS, Smith I. Amino acids and related compounds. In : Smith I, Seakins JWT (Eds.), Chromatographic and electrophoretic techniques, vol.1, 4th edition. London : William Heinemann. 1976; 183-217.
- Gulati S, vaswani M, Kalra V, Kabra M, Kaur M. An approach to neurometabolic disorders by a simple metabolic screen. Indian Pediatrics 2000; 37: 63-69.
- Christopher R, Sankaran BP. An insight into the biochemistry of inborn errors of metabolism for a clinical neurologist. Ann Indian Acad Neurol 2008; 11: 68-81.
- Mattingley JM, Paper chromatography of urinary amino acids. A 30 year survey of dietary influences on the normal pattern, and patients' results, Biomed Chromatogr 1986; 1 (3): 95-100.
- Jailkhani R, Patil VS, Laxman HB, Shivashankar AR, Kulkarni SP, Ravindra MS. Selective screening for inborn errors of metabolism in children: single centre experience from Karnataka. Journal of Clinical and Diagnostic Research, 2008; 2: 952-958
- Fenton WA, Gravel RA, Rosenblatt DS. Disorders of propionate and methylmalonate metabolism. In : Scriver CR, Beaudet AL, Sly WS, Valle D (Eds.), The metabolic and molecular bases of inherited disease, 8th edition. New York: Mc-Graw Hill 2001; 2165-2193.
- Bhatt C, Misra Z, Goyel N. Detection of inherited metabolic diseases in children with mental handicap. Ind J of Clin Biochem 2008; 23 (1): 10-16.
- Bijarnia S, Puri RD, Ruel J, Gray GF, Jenkinson L, Verma IC. Tyrosinemia type I-Diagnostic issues and prenatal diagnosis. Indian J Pediatr 2006; 73 (2): 163- 165.
- Songa Y, Lia B, Hu Haoa et al. Selective screening for inborn errors of metabolism and secondary methylmalonic aciduria in pregnancy at high risk district of neural tube defects: A human metabolome study by GC-MS in China. Clin Biochem 2008; 41 (7-8); 616- 620.
- Raghuveer TS, Garg U, Graf WD et al. Inborn errors of metabolism in infancy and early childhood: and update. Am fam physician, 2006; 73 (11): 1981-1990.
- Mudd HS, Braveman N, Pomper M et al. Infantile hypermethioninemia and hyperhomocystinemia due to high methionine intake: A diagnostic trap. Mol Genet Metab 2003; 79 (1): 6-16.
- Perry TL, Hardwick DF, Dixon GH, Dolman CL, Hansen S. Hypermethioninemia: A metabolic disorder associated with cirrhosis, islet cell hyperplasia and renal tubular degeneration, Pediatrics 1965; 36 (2): 236-250.