Review Article - Otolaryngology Online Journal (2020) Volume 10, Issue 6
SARS-CoV-2: A Systematc Review
Poonam Goel*, Vaibhav GuptaMaulana Azad Institute of Dental Sciences, New Delhi, India
- *Corresponding Author:
- Dr. Poonam Goel, MDS
Research Fellow, Department of Academic Research Section
Maulana Azad Institute of Dental Sciences, New Delhi, India
Tel: +8920898693
E-mail: dr.poonam1187@gmail.com
Received: September 22, 2020; Accepted: September 23, 2020; Published: September 30, 2020
Abstract
Introducton: SARS-CoV-2 outbreak in China has spread its roots to almost all the countries around the world and infected lakhs of individuals. To summarize the demographical, clinical, laboratory, radiographic features, comorbidites, and treatment strategies of confrmed SARS-CoV-2 infecton, the systematc review was designed.
Sources: PubMed and Google Scholar artcles were searched for English-language artcles published between January and April 2020.
Data: A total of 156 artcles were identfed by the literature search from which53full text artcles met the inclusion criteria. Afer quality assessment using variouschecklists, 15artcles were included.
Study selecton: Artcles included in the review presented studies from four contnents of world with majority from China Mean age of patents with corona infecton was 49.3 years, with 56% males and 44% females. Along with this 8% of the individuals were reported with smoking. Major clinical symptoms were fever, dry cough, shortness of breath, fatgue and around 27.5% patents presented with comorbidites. Choice of treatment was antviral therapy along with symptomatc therapy.
Conclusion: Itcan help clinician to diagnose and plan treatment for SARS-CoV-2 patent which appears to be highly communicable from the existng epidemiological data with varying fatality rate. More rapidly the cases are detected, isolated, and traced; the more efcacious will be the preventon strategies for any country to evade community transmission.
Keywords
Corona, Pandemic, Antiviral, Fever, Cough, Virus
Introduction
Since the dawn of severe acute respiratory syndrome Coronavirus (SARS‐CoV) in 2002 with its spread through 32 countries, the world has experienced the wave of Middle East respiratory syndrome Coronavirus (MERS‐CoV) and now, the unnerving novel Coronavirus (2019-nCoV) [1]. Corona viruses, the largest known RNA virus genomes are basically the enveloped, non-segmented, positive‐sense single‐stranded known to root diseases in mammals as well as humans. Among the four genera of coronavirus subfamily α, β, ɣ, and δ, α‐ and β‐CoVs cause human infections. CoVs are supposed to be common human pathogens, and 30% to 60% of the Chinese population was already known to be positive for anti‐CoV antibodies [1]. β-CoV group umbrella both the SARS-CoV and MERS-CoV [2,3]. Moreover, the phylogenetic analysis showed that 2019-nCoV viral genome that scouted in Wuhan also belonged to the β-CoV [4]. Being 79% similar nucleotide sequence to SARS-CoV and 50% similar to MERS-CoV, 2019-nCoV can equally cause the disastrous infection and with a much faster spread than the two other corona viruses [5,6].
An outbreak of viral pneumonia cases of unknown cause was attested by health authorities in Wuhan, the capital of Hubei province, China during the end of 2019 and the beginning of 2020. Huanan Seafood Wholesale Market which vended live animals, seemed to be one of the source as many of the infected cases were found to have recent visit; consequently making the origin of this unknown virus as zoonotic [1,7]. The pneumonia infection has rapidly spread from Wuhan to other provinces of china and crossing borders to other 114 countries. Over this global pneumonia outbreak, World Health Organization has declared a public health emergency of international concern on 30th January 2020 [5].
On 11th February 2020, WHO named the novel viral pneumonia as “Corona Virus Disease (COVID19)”, while the International Committee on Taxonomy of Viruses (ICTV) suggested this novel Coronavirus name as “SARS-CoV- 2” [8]. Since December 2019, SARSCoV- 2 has caused over 84,180 cases of COVID-19 in China, including 4,642 deaths, as of 18 April 2020. The epidemic has been spreading to almost all the countries around the world, with more than 23 lakh confirmed cases and more than 1.6 lakh deaths as reported by World Health Organization (WHO) on 20 April.
Transmission occurs chiefly via respiratory droplets from cough, sneeze and contact transmission. Carriers of SARS-CoV-2 include infected COVID-19 patients, asymptomatic patients and patients in their incubation period. The incubation period has been gaugedto 5 to 6 days on average, but may extend to 14 days [9,10]. Researches stanch that this novel epidemic doubled in about every seven days, while the basic reproduction number (R0 - R naught) is 2.2. To a layman this means that on an average single patient transmits this infection further to superfluous 2.2 individuals. However, the estimations of the R0 of the SARS-CoV epidemic in 2002-2003 were made approximately as 3[11].
Since the appearance of SARS-CoV-2, few studies related to Coronavirus including case reports, retrospective studies have been published in past four months [12-15]. Most of these articles have reported clinical outcomes, investigations, treatment/ response and complications. But a comprehensive systematic review with amalgamation of all the details has not been done till date. Hence the systemic review is planned with following objectives to understand COVID-19 and consider how to flatten the curve.
Objectives
• To summarize the clinical, laboratory, and radiographic features of SARS-CoV-2 reported in available literature.
• To examine the various treatment regime followed for SARS-CoV-2 and to assess their effectiveness.
• To assess the outcome of SARS-CoV-2 cases, including risk factors, the proportion of patients requiring ICU and the fatality rate.
• To assess the prevalence of comorbidities among SARS-CoV-2confirmed cases.
Methodology
Data sources and search strategy
A systematic literature search was conducted to identify English-language published peerreviewed articles with demographical, clinical features, comorbidities, laboratory investigations, radiographic features and treatment strategies of confirmed SARS-CoV-2 infection. Studies published from January 1, 2020 until April 10, 2020 wassearched on researchportals like Google Scholar andMEDLINE (through PubMed).Following search terms relating to “COVID-19”,“coronavirus 2019”, “Novel Coranavirus”, “COVID-19 clinical and radiographic features”, “COVID-19 lab investigation”, “COVID-19 prevention and treatment”, “COVID-19 complications”. All records electronically identified were scanned by title, abstract and/ or the keywords by both authors and the full text of all the reports considered potentially relevant was obtained. The reference lists from the studies retrieved by this search were reviewed and any relevant references followed up.
Study selection
Studies were included
1) If details of at least one patient with corona infection given,
2) If clinical features, laboratory, radiographic investigations of SARS-CoV-2 were given,
3) All hospital based studies were included, providing treatment to cure the patient from COVID-19.
Study review and data extraction
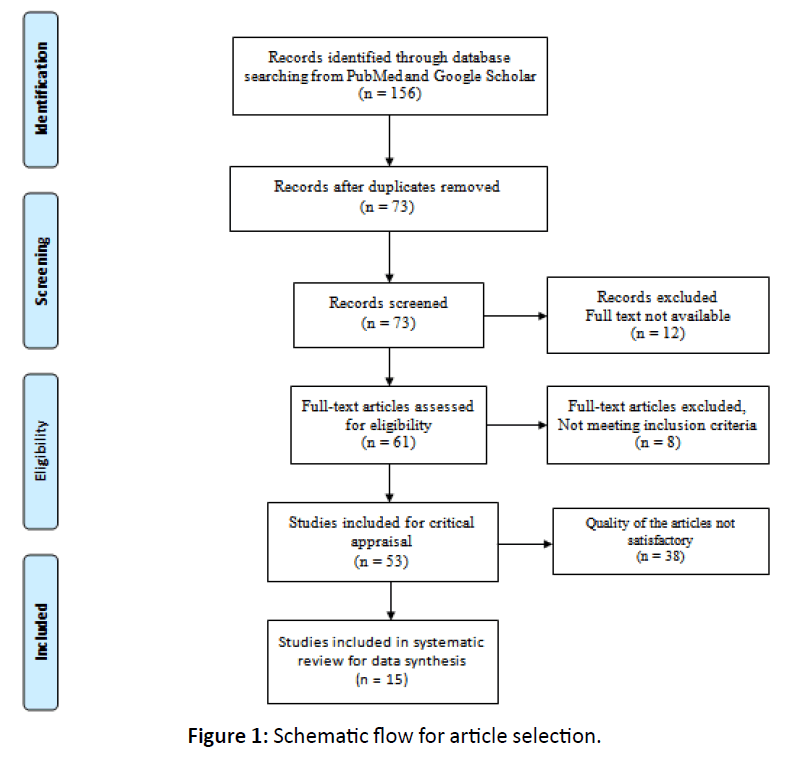
The baseline searches were carried out by all the authors. The selection of studies on the basis of title, keywords and abstract and decisions about eligibility were carried out independently by the review authors. Studies not following inclusion criteria (according to study design, subjects) were discarded. The full text of all the studies considered potentially relevant for inclusion was obtained. If relevant information according to inclusion criteria was not available in the abstract or if the title was relevant but the abstract was not available, the full text of the report was obtained. Each study was reviewed and critically appraised for quality according to a set of validity criteria as per STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [16] and CARE (CASE REPORT) guidelines [17]. If all criteria were met, a maximum score of 22 was assigned. Studies scored 12 or more according to validity criteria were included in the study as good quality articles. All information and data was recorded independently by all the authors and no disagreements were there. Total 156 publications were retrieved, of which 73 publications were identified based on the title and abstract. From these 12 were excluded because full text was not available, 8 publications didn’t meet inclusion criteria and 38 were of poor quality as per STROBE or CARE guidelines (Figure 1). Finally 15 studies fulfilled our criteria, and from each article all the information pertaining to patient’s demographic details, comorbidities, clinical, laboratory, and radiographic features and provided treatment were extracted and recorded in Microsoft Excel 2010 software.
Results
In this review, 15 studies are included published between January and April, 2020 with representation from four continents of world with majority from China (Table 1). Publications from European region were also selected but due to stringent inclusion criteria it was excluded. Characteristics of 7770 participants were studied in these 15 publications. Mean age of patients with corona infection was 49.3 years, with 56% males and 44% females. Along with this 16.5% of the individuals were reported with smoking habit (Table 2). Around 27.5% patients presented with comorbidities like hypertension (13.7%), diabetes (9.8%) and cardiovascular issues (11.5%) as the substantial ones (Table 3).
Table 1. Details of articles related to Coronavirus included for review.
| Sl. No | Author | Journal | Country | Study Type | N | Ref: |
|---|---|---|---|---|---|---|
| 1 | Dawei Wang et al. | JAMA | China | Case series | 138 | 12 |
| 2 | Nanshan Chen et al. | Lancet | China | Retrospective | 99 | 24 |
| 3 | Matt Arentz et al. | JAMA | Washington | Case series | 21 | 13 |
| 4 | Jian Wu et al. | Clinical Infectious Diseases | China | Retrospective | 80 | 18 |
| 5 | Fei Zhou et al. | Lancet | China | Retrospective cohort | 191 | 26 |
| 6 | Xiao-Wei Xu et al. | BMJ | China | Retrospective | 62 | 31 |
| 7 | Kui Liu et al. | Chinese Medical Journal | China | Case series | 137 | 25 |
| 8 | Wei-jie Guan et al. | medRxiv | China | Retrospective | 1099 | 22 |
| 9 | Michael Chung et al. | Radiology | China | Case series | 21 | 32 |
| 10 | Yueying Pan et al. | European Radiology | China | Retrospective | 63 | 33 |
| 11 | De Chang et al. | JAMA | China | Retrospective | 13 | 34 |
| 12 | COVID-19 Australia Team | Epidemiology Report | Australia | Retrospective | 5805 | 35 |
| 13 | Moran Ki et al. | Epidemiology and Health | Korea | Case series | 28 | 36 |
| 14 | Juan Pablo Escalera-Antezana et al. | Formosan Medical Association | Bolivia | Retrospective | 12 | 37 |
| 15 | Eu Suk Kim et al. | Journal of Korean Med Science | Korea | Retrospective | 28 | 15 |
Table 2. Demographic details and clinical features summary.
| Variable | Mean/ percentage | No. of studies |
|---|---|---|
| Age | 49.3 | 15 |
| Gender | Males -56%, Females-44% | 15 |
| Smoking | 16.5% | 2 |
| ICU | 14.4% | 9 |
| Clinical symptoms | ||
| Fever | 78.3 | 14 |
| Cough | 55.3 | 14 |
| Dysponea | 49.3 | 8 |
| Sore throat | 27 | 7 |
| Myalgia | 24.7 | 12 |
| Fatigue | 48.4 | 9 |
| Sputum production | 26.5 | 7 |
| Headache | 24.3 | 11 |
| Nasal congestion | 15.9 | 3 |
| Haemoptosis | 0.3 | 3 |
| Diarrhoea | 1 | 9 |
| Anorexia | 0.7 | 1 |
| Pharyngalgia | 0.6 | 2 |
| Nausea | 1 | 6 |
| Dizziness | 0.2 | 1 |
| Vomiting | 0.9 | 6 |
| Abdominal pain | 0.1 | 4 |
| Confusion | 0.1 | 1 |
| Rhinorrhea | 1.2 | 5 |
| Eye congestion | 0.1 | 2 |
Table 3. Comorbidities and radiographic features summary.
| Variable | Percentage | No. of studies |
|---|---|---|
| Comorbidities | 27.5 | 11 |
| Hypertension | 13.7 | 7 |
| CVS | 11.5 | 8 |
| Diabetes | 9.8 | 10 |
| Cancer | 1.4 | 7 |
| COPD | 4.4 | 8 |
| Asthma | 0.1 | 3 |
| Chronic liver diseases | 5.6 | 7 |
| Chronic kidney diseases | 4.5 | 7 |
| HIV | 0.03 | 1 |
| Digestive system disease | 0.2 | 2 |
| CNS | 0.2 | 4 |
| Obstructive sleep apnea | 0.1 | 1 |
| Immunosupression | 0.1 | 2 |
| Rheumatologic disease | 0.01 | 1 |
| Hypothyroidism | 0.01 | 1 |
| Community Acquired pneumonia | 0.01 | 1 |
| Radiographic Features | ||
| Chest Ray Unilateral Pneumonia | 47.1 | 7 |
| Chest Ray Bilateral Pneumonia | 64.5 | 11 |
| Ground-glass opacity | 62.3 | 11 |
| Pleural effusion | 0.2 | 1 |
| Peribronchial thickening | 0.2 | 2 |
| Focal consolidation | 2.1 | 5 |
| Pulmonary edema | 0.02 | 1 |
| Intestinal abnormalities | 2 | 1 |
Most common symptoms reported are fever (78.3%), cough (55.3%), dyspnea (49.3%), fatigue (48.4%), sore throat (27%) followed by headache (24.3%), nasal congestion (15.9%) and few more as shown in table 2. Laboratory investigations revealed decreased albumin (61.7%), lymphopenia (39.8%), High C-reactive protein (50.2%), increased LDH (48.4%) and increased AST (24.1%) as the major findings (Table 4) with bilateral pneumonia (64.5%), ground glass opacity (62.3%) and unilateral pneumonia (47.1%) as major radiographic findings (Table 4).
Table 4. Laboratory features summary.
| Variable | Percentage | No. of studies |
|---|---|---|
| Leococytosis | 2.4 | 8 |
| Leucopenia | 16.5 | 9 |
| Lymphopenia | 39.8 | 11 |
| Thrombocytopenia | 5.8 | 5 |
| Low Hb | 11.8 | 4 |
| High AST | 24.1 | 7 |
| High ALT | 23.7 | 7 |
| High creatinine | 7.8 | 6 |
| High creatine kinase | 11.1 | 7 |
| High LDH | 48.4 | 8 |
| Decreased albumin | 61.7 | 3 |
| High bilirubin | 11.3 | 3 |
| High interleukin-6 | 8.3 | 3 |
| High procalcitonin | 9.1 | 7 |
| High C reactive protein | 50.2 | 6 |
| Elevated ESR | 33.9 | 3 |
Nine studies reported that 14.4% patients were admitted to intensive care unit (ICU) due to complications like acute respiratory distress syndrome (ARDS) in 28.4%, ventilator-associated pneumonia in 11.1%, acute cardiac injury in 9.4%, acute hepatic injury in 6.4% and few more listed in table 5. Around 54% were discharged and 7.8% had fatal outcomes.
Table 5. Summary of the treatment provided and the complications among the selected articles.
| Variable | Percentage | No. of studies |
|---|---|---|
| Treatment provided | ||
| Antiviral | 48.7 | 11 |
| Antibiotics | 42.3 | 8 |
| Antifungal | 4.8 | 2 |
| Vasopressor | 6.4 | 2 |
| Oxygen | 26.5 | 5 |
| Intravenous immunoglobulin therapy | 23.6 | 5 |
| Glucocorticoid therapy | 35.3 | 7 |
| Continuous kidney replacement therapy | 12.4 | 4 |
| Extracorporeal membrane oxygenation | 15.2 | 4 |
| Invasive mechanical ventilation | 12.7 | 8 |
| Noninvasive ventilation | 13.4 | 8 |
| Traditional chinese medicine | 0.2 | 1 |
| Complications | ||
| Shock | 5.1 | 4 |
| Acute cardiac injury | 9.4 | 3 |
| ARDS | 28.4 | 8 |
| Arrhythmia | 2.1 | 1 |
| Acute renal injury | 5.8 | 5 |
| Acute hepatic injury | 6.5 | 2 |
| Acute respiratory injury | 3.9 | 4 |
| Heart failure | 0.8 | 1 |
| Sepsis | 2.1 | 1 |
| Seizures | 0.5 | 1 |
| Ventilator-associated pneumonia…… | 11.1 | 2 |
| DIC - Disseminated intravascular coagulation | 0.1 | 1 |
| Secondary infection | 5.4 | 4 |
| Outcome | ||
| Hospitalization | 38 | 15 |
| Discharge | 54.2 | 14 |
| Death | 7.8 | 8 |
In most of the studies (73%), choice of treatment was antiviral therapy including oseltamivir, ritonavir, lopinavir and ganciclovir along with antibiotics in 53% and antifungal in 13% of studies. Along with this intravenous immunoglobulin, corticosteroids, continuous kidney replacement therapy, extracorporeal membrane oxygenation and mechanical ventilation (both invasive and noninvasive) were also used as per the need of the patients (Table 5). One of the studies even tried Chinese medicine too [18].
Discussion
SARS-CoV-2 outbreak has been established as a public health emergency with more than 200 countries snowed under this pandemic. Within last four months it has made around 6 lakh in USA, 1.7 lakh in Spain, 1.6 lakh in Italy, 1.25 lakh in Germany and around ten thousand in India as disease-ridden. Even the number of confirmed death increased to thousands in USA, UK, Spain, Italy, Germany, and France whereas in India there were 395 reported deaths [19]. To get a global outline of this desolation, studies from all over the world with presentation from each continent were included. And through this review it has been tried to calculate the fatality rate. Rendering to contemporary statistics, the fatality rate (cumulative deaths divided by cumulative cases) of COVID-19 was found to be 0.09% to 9.84%, contingent on diverse regions of world (USA 1.8%, Italy 9.84%, China 4.1%, India 2.4%, South Africa 0.09%), which appears to be lower than that of SARS ≈10% and MERS ≈39% and higher than that of seasonal influenza (0.01% to 0.17%) [10,20]. High case-fatality risks is comprehended at the peak of local epidemics, which may be appropriate in highincome countries with limited surge capacity in hospital services [21].
The majority of COVID-19 patients exist as mild asymptomatic cases. According to current researches, the section of severe COVID-19 cases in China fall upto 15% to 25% [10,22]. Representative clinical symptoms are fever and dry cough, with shortness of breath, fatigue, with someunusual symptoms, such as sore throat, muscle pain, pharyngalgia, confusion, anorexia, headache, eye congestion, diarrhea, nausea and vomiting. In presence of comorbidities like hypertension, nervous system, diabetes, cardiovascular system and digestive system it is challenging to diagnose the coronavirus disease as various initial symptoms gets encased. The median interval from the onset of initial symptoms to dyspnea or significant symptom aggravation was seven days, ranging between day one (ie, appearance of acute respiratory distress syndrome) up to 20 days, which was consistent with previous reports. Day one involves fever, fatigue, muscle pain, dry cough leading to difficulty breathing on day five. On 10th day patients may experience abdominal pain. The complete recovery and hospital discharge usually takes two and half weeks [23-25]. Some of the patients subsequently developed ARDS, ventilator-associated pneumonia, acute cardiac or hepatic injury and required care in the intensive care unit [13,26]. Smoking is associated with the negative progression and adverse outcomes of COVID-19 [27]. In review 16.5% of the individuals were reported with smoking habit [15,22].
The early stage symptoms are nonspecific. Differential diagnosis should include the likelihood of a comprehensive array of common respiratory disorders counting both infectious like Adenovirus, Influenza, Human metapneumovirus (HmPV), Parainfluenza, Respiratory syncytial virus (RSV), Rhinovirus (common cold) and non-infectious like e.g., vasculitis, dermatomyositis. For alleged cases, rapid antigen detection, and other investigations should be espoused for gaging the prevalent respiratory condition [11].
Since emergence of SARS-CoV-2, scientists around the world are conducting studies to find the most effective drug for its treatment. Several drugs such as chloroquine, lovinavir, ritonavir, arbidol, remdesivir, oseltamivir and favipiravir are currently undergoing evaluation to test their effectiveness in the coronavirus treatment. Being a broad-spectrum antiviral, IFN-α is used to treat hepatitis, and has also been reported to inhibit SARS-CoV in-vitro replication. Lopinavir/ ritonavir in combination with other drugs is known to treattreat adults and children over 14 days of age who are infected with HIV-1. Chloroquine,besides acting as antimalarial drug, was also found to be a prospective broad-spectrum antiviral in 2006. It was found to block SARS-CoV-2 infection at particularly at low micromolar concentration. Arbidol, another antiviral can be used to treat influenza virus [28]. And even in the review, almost half of the patients received some kind of antiviral therapy as a part of treatment in 73% of the studies along with supportive symptomatic treatment [12,18,24-26]. Chloroquine/ hydroxychloroquine are advised as a prophylactic drug for SARS-CoV-2 infection especially for high risk population (asymptomatic health care workers involved in the care of suspected or confirmed cases of COVID-19 and asymptomatic household contacts of laboratory confirmed cases) [29].
Results obtained from this systematic review may help clinicians in diagnosing patients using clinical, laboratory and radiographic findings among SARSCoV- 2 suspected patients visiting hospital for checkups. Along with these characteristics, vigilant medical history to be recorded along with recent international travel and contact with any confirmed case. Review results can also help the clinician to plan the treatment depending upon the patient symptoms and assessing its effectiveness. Further clinical trials are required to narrow down to the most effective drug for Coronavirus treatment.
Limitations
Bias could have been introduced due to restriction of studies to English language, relevant data included in other journals would have been missed because of limited access (high registration amount) and also MEDLINE, EBSCO and Google Scholar database do not contain all research reports. Furthermore, most of the studies included were from china which may not depict true geographic representation and may lack generalizability.
This systematic review is written as per the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [30-37].
Conclusion
SARS-CoV-2 appears to be highly communicable from the existing epidemiological data with varying fatality rate. Due to lack of drug trials, Coronavirus outbreak by a novel virus has limited the treatment options. In this baffling time, public health measures like social distancing, home quarantine, proper hygiene measures like hand washing along with mandatory vigorous community testing needs to be followed. More rapidly the cases are detected, isolated, and traced; the more efficacious will be the prevention strategies for any country to evade community transmission.
Conflict of Interest: Nil
Source of Funding: Nil
References
- Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020; 92:424-32.
- Al-tawfiq JA, Zumla A, Memish ZA. Coronaviruses : severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus in travelers. Trop Travel Dis. 2014; 27:411-7.
- Song Z, Xu Y, Bao L, et al. From SARS to MERS, Thrusting Coronaviruses into spotlight. Viruses. 2019; 11:59.
- Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579: 270-3.
- Peng X, Xu X, Li Y, et al. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020; 12(1):1-6.
- Tong Y, Ph D, Ren R, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020; 382(13):1199-207.
- Mathur N, Tyagi S, Dwivedi V, et al. Dental considerations amidst covid-19 scare. Int J Med Biomed Stud. 2020; 4(3):141-5.
- Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses-a statement of the Coronavirus Study Group.
- Rio C del, Malani PN. 2019 Novel Coronavirus - Important Information for Clinicians. J Am Med Assoc. 2020; 323(11):1039-40.
- Meng L, Hua F, Bian Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J Dent Res. 2020; 1:1-7.
- Cascella M, Rajnik M, Cuomo A, et al. Features, Evaluation and Treatment Coronavirus (COVID-19).
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020; 323(11): 1061-9.
- Characteristics and Outcomes of 21 Critically Ill Patients with COVID-19 in Washington State. 2020; 4720:2019-21.
- Cheng S, Chang Y, Chiang Y-LF, et al. First case of Coronavirus Disease 2019 (COVID-19) pneumonia in Taiwan. J Formos Med Assoc. 2020.
- Kim ES, Chin BS, Kang CK, et al. Clinical Course and Outcomes of Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection : a Preliminary Report of the First 28 Patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020; 35(13): e142.
- STROBE Statement-checklist of items that should be included in reports of observational studies. 2007.
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Heal Med. 2013; 2(5): 38-43.
- Wu J, Liu J, Zhao X, et al. Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province : A Multicenter Descriptive Study. 2020.
- Numbers SIN. Coronavirus Disease 2019 (COVID-19) Situation Report - 85. 2020.
- Koh D, Sng J. Lessons from the past: perspectives on severe acute respiratory syndrome. Asia Pacific J Public Heal. 2010; 22(3): 132S-6S.
- Wilson N, Kvalsvig A, Barnard LT, et al. Case-Fatality Risk Estimates for COVID-19 Calculated by Using a Lag Time for Fatality. Emerg Infect Dis. 2020; 26.
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China Wei-jie. Med Rxiv. 2020.
- Jiang F, Deng L, Zhang L, et al. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med. 2020:1-5.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China : a descriptive study. Lancet. 2020; 395(10223): 507-13.
- Liu K, Fang Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China : a retrospective cohort study. Lancet. 2020; 395(10229): 1054-62.
- Vardavas CI, Nikitara K. COVID-19 and smoking : A systematic review of the evidence. Tob Induc Dis. 2020; 18: 20.
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). 2020; 14(1): 58-60.
- Welfare G. Ministry of Health and Family, COVID-19 India. 2020.
- David Moher, Alessandro Liberati, Jennifer Tetzlaff, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). PLoS Med. 2009; 6(6): e1000097.
- Xu X, Wu X, Jiang X, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan , China : retrospective case series. 2020; 2019:1-7.
- Chung M, Bernheim A, Mei X, et al. CT Imaging Features of 2019 Novel Coronavirus. Radiology. 2020; 295(1): 202-7.
- Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia ( 2019-nCoV ): a study of 63 patients in Wuhan , China. Eur Soc Radiol. 2020; 1-4.
- Chang D, Lin M, Wei L, et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients outside Wuhan, China. JAMA. 2020; 323(11):1092-3.
- Team C-19 NIRS. COVID-19, Australia : Epidemiology Report 10. Commun Dis Intel. 2020; 44:1-21.
- Ki M, Force T. Epidemiologic characteristics of early cases with 2019 novel Coronavirus (2019-nCoV) disease in Korea. Epidemiol Health. 2020; 42:e2020007.
- Escalera-antezana JP, Lizon-ferrufino NF, Maldonado-alanoca A, et al. Clinical features of the first cases and a cluster of Coronavirus Disease 2019 (COVID-19) in Bolivia imported from Italy and Spain. Travel Med Infect Dis. 2020.