Research Article - Biomedical Research (2018) Volume 29, Issue 11
Salvadora persica leaf aqueous extract attenuates hyperglycemia and hyperlipidemia in alloxan induced diabetic male rats
Haddad El Rabey A1,2*, Fahad Almutairi M1, Abdulbasit Al-Sieni I3, Madeha Al-Seeni N3, Mohammed Al-Duais A1,4, Mohamed Sakran I1,5 and Abuelgassim Abuelgassim O6
1Department of Biochemistry, Faculty of Science, University of Tabuk, Tabuk, Kingdom of Saudi Arabia
2Department of Bioinformatics, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Egypt
3Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia
4Department of Chemistry, Faculty of Science, Ibb University, 70270 Ibb, Yemen
5Department of Chemistry, Faculty of Science, Tanta University, Egypt
6Department of Biochemistry, College of Science, King Saud University, Riyadh, Saudi Arabia
- *Corresponding Author:
- Haddad El Rabey A
Department of Biochemistry
Faculty of Science
University of Tabuk, Tabuk
Kingdom of Saudi Arabia
Accepted on April 10, 2018
DOI: 10.4066/biomedicalresearch.64-18-549
Visit for more related articles at Biomedical ResearchAbstract
Background: Herbal medicine has been widely used in controlling diabetes and obesity for avoiding their complications.
Objectives: The anti-diabetic, antioxidant and anti-lipidemic activity of Salvadora persica leaf aqueous extract was tested in alloxan induced diabetic male rats.
Methods: 24 male rats were divided into 4 groups; negative control (G1), diabetic positive control (G2) and two diabetic groups (G3 and G4) supplemented with aqueous extract of S. persica at a dose of 100 and 200 mg/0.5 ml water/100 g body weight, respectively for 28 d.
Results: The positive control diabetic group showed increase in blood sugar, liver enzyme activity, kidney parameters, lipid peroxidation, immunoglobulines, total cholesterol, triglycerides, low density lipoprotein, very low density lipoproteins and decrease in alpha amylase, antioxidants and high density lipoproteins. Treating the diabetic rats in G3 and G4 with S. persica leaf aqueous extract nearly restored all biochemical and histological changes nearly to the normal as in the control negative.
Conclusion: S. persica leaf aqueous extract has hypoglycaemic, antioxidant and hypolipidemic activity in alloxan induced diabetic male rats. It restored all biochemical parameters and the injured kidney, liver and pancreas tissues nearly to the normal as in the negative control group. The high dose of S. persica leaf aqueous extract was more effective than the low dose.
Keywords
Anti-diabetic, Hypolipidemic, Salvadora persica, Leaf, Alloxan, Rats.
List of Abbreviations
ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; BWG: Body Weight Gain; BWG%: Body Weight Gain ratio (Percent); Cat: Catalase; FER: Food Efficiency Ratio; FI: Food Intake; G1: The 1st group was the normal untreated control group fed fat rich diet; G2: The 2nd group was the positive control diabetic group and was fed fat rich diet; G3: The 3rd group, was supplemented with aqueous extract of S. persica by stomach tube at a dose of 100 mg/0.5 ml distilled water/100 g body weight per day; G4: The 4th group was supplemented with aqueous extract of S. persica by stomach tube at a dose of 200 mg/0.5 ml distilled water/100 g body weight per day; GGT: Gamma-Glutamyl Transferase; GST: Glutathione S-Transferases; HDL: High Density Lipoprotein; H and E: Hematoxylin and Eosin; LDL: Low Density Lipoprotein cholesterol; LSD: Least Significant Difference; MDA: Malondialdehyde; SOD: Superoxide Dismutase; S. persica: Salvadora persica; SPSS: Statistical Program for Sociology Scientists; TC: Total Cholesterol; TG: Triglyceride; VLDL: Very Low Density Lipoproteins cholesterol; UA: Uric Acid.
Introduction
Salvadora persica L. (toothbrush tree) is a perennial tree belongs to the family Salvadoraceae from which, meswak, a chewing stick is prepared from its stings and roots for tooth cleaning in Arabian countries. Meswak is widely used in Islamic countries due to the fact that it is a part of Islam’s religious practice and its use in maintaining the dental hygiene and ultimately the potential and safely use as dental remedy [1]. Stem decoction of S. persica showed hypoglycemic effects, increased plasma Immunoreactive Insulin (IRI) and incremented oral-glucose tolerance in normal rats [2].
Leaves of S. persica are eaten as a vegetable in the eastern tropical Africa and are used in the preparation of a sauce, tender shoots and are eaten as salad [3]. Recent investigations showed that S. persica has hypoglycemic and hypolipidemic activity [4-6]. S. persica has also antimicrobial activity that enabled it to be used toothpastes manufacture in different countries [7,8]. Salvadora persica twig powder showed hypoglycemic, antioxidant and hypolipidemic activity in alloxan induced diabetic rats [9].
In the present study, the anti-diabetic, the antihypercholesterolemic and the antioxidant activity of the aqueous extract of S. persica leaf was tested in alloxan induced diabetic male rats.
Materials and Methods
Chemicals used in this study are of analytical grade.
Plant materials
Leaves of Salvadora persica tree were collected from Jeddah, Saudi Arabia. The leaves were washed with water, dried and then milled using mixer for aqueous extract preparation.
The fat rich diet
The fat rich diet was prepared according to Knapka et al. [10] by mixing the following constituents: 16% casein, 10% corn oil, 4% N.N cellulose, 4% salt mixture, 1% vitamin mixture, 0.2% choline chloride, 0.2% DL. methionine and 64.6% corn starch.
Preparation of the aqueous extract of S. persica leaf
The aqueous extract was prepared according to the method of Sharma et al. [11]. Briefly, the dry leaves were grinded by grinder. 100 g of the leaf powder were macerated in 1 litre purified water and kept in dark for 48 h at room temperature. 1% chloroform was added to avoid microbial growth. The solution was filtered by Whatman Filter Paper No. 1 and the filtered extract was then dried in a rotary evaporator to obtain a dark brown powdery extract (12.5% w/w).
Experiment design
Twenty four male albino rats weighing 165-190 g (two months age) were obtained from King Fahd Center for medical research, King Abdulaziz University. The protocol of this study was approved by the Ethical Committee of King Abdulaziz University. Animals were housed 6 per polycarbonate cage. Cages, bedding, and glass water bottles were replaced twice per week. The stainless steel feed containers were changed once a week. The 24 rats were divided as follows: 6 rats for the first group (G1) the normal untreated control group fed fat rich diet. The other 18 rats were intraperitoneally injected with a single dose of alloxan monohydrate (150 mg/kg between dissolved in distilled water) after fasting for 12 h to induce diabetes [12]. After 5 d of injection, rats with blood glucose higher than 200 mg/dl in the fasting state were considered as being diabetic and were divided into 3 groups; the second group (G2) was the positive control diabetic group and were fed fat rich diet. The 3rd (G3) and the 4th (G4) groups were supplemented with aqueous extract of S. persica by stomach tube at a dose of 100 and 200 mg/0.5 ml distilled water/100 g body weight per day, respectively for 28 d.
Dissection and blood collection
At the end of the experiment, animals were fasted for 14-16 h after their last feeding and blood was collected from the heart of Dimethyl-ether pre-anaesthetized rats in plain tubes for biochemical analysis. Blood serum was obtained by centrifugation at 1000 rpm for 10 min at room temperature, and then stored at -20°C until analysis was performed. Animals were sacrificed by cervical dislocation, and then the abdomen was dissected and the target organs (the liver, the two kidneys and the pancreas) were rapidly excised. A piece of liver (100 mg) was rinsed in saline and then saved in ice-cold for antioxidant enzymes and lipid peroxidation estimation in liver tissue homogenate. The rest of the liver, one kidney and the pancreas were rinsed in saline for few seconds, and then kept in 10% formalin for histopathological investigations.
Preparation of tissue homogenate
The ice-cold liver sample (100 mg) was homogenized in 0.1M Tris-HCl buffer (pH 7.4) with a Teflon homogenizer at 4°C. The homogenate was centrifuged at 12000 rpm for 5 min to remove the debris, and then the supernatant was used for estimation of the antioxidant enzymes and lipid peroxide.
Determination of fasting blood sugar (FBS)
Human kit (Germany) was used in determination of fasting blood sugar according to the method of Trinder et al. [13].
Glycated hemoglobin (HbA1c)
Glycated hemoglobin (HbA1c) was determined in whole blood according to the method described in variant [14] using kit supplied by Pointe Scientific, Inc., United States.
Serum α-amylase activity
Serum α-amylase activity was determined according to the method described by YingFoo et al. [15] using a kit supplied by Human, Germany.
Determination of immunoglobulins (Ig)
Immunoglobulins (IgA, IgM and IgG) were estimated in the serum according to Berne [16] using commercially available kits from Genway Biotech (USA).
Liver enzymes
Serum Alanine Transaminase (ALT) and Alkaline Phosphatase (ALP) were estimated spectrophotometrically according to the method of Thefeld et al. [17] and Schlebusch et al. [18], respectively using Human Kit (Germany) according to the instruction of the supplier. Serum Aspartate Aminotransferase (AST) was estimated spectrophotometrically according to the method of Thefeld et al. [17] using Swemed Diagnostics kit (India). Gamma-glutamyl transferase (GGT) activity was measured in serum using a modified kinetic method of Gowenlock et al. [19] using kit from Spinreact, Spain.
Kidney function
Serum urea and Uric acid were estimated spectrophotometrically as described by Fawcett et al. [20], Fossati et al. [21], respectively using Human kit (Germany). Serum creatinine was estimated spectrophotometrically as described by Tietz [22] using Moody International creatinine kit (UKAS, Germany) according to the instruction of the supplier.
Estimation of serum electrolytes (Na+ and Ca++)
Serum Na+ and Ca++ were estimated by colorimetric methods using Electrolytes Test Kit (India) according to Schoenfeld et al. [23,24], respectively according to the instruction of the supplier.
Estimation of bilirubin
Spectrum kit (Germany) was used in determination of total bilirubin spectrophotometrically according to the method described by Balistreri et al. [25].
Lipid profile
Serum Total Cholesterol (TC) and Triglycerides (TG) were determined by colorimetric methods as described by Young [26] using Spinreact Kit (Spain) according to the instruction of the supplier. Serum High Density Lipoprotein (HDL) was estimated according to the colorimetric method of Naito [27] using Spinreact Kit (Spain) according to the instruction of the supplier. Serum LDL and VLDL were calculated according to the equation of Srivastava et al. [28] as follows: LDL=TC- (HDL+TG\5) and VLDL=TC-(LDL+HDL).
Antioxidants and lipid peroxidation
The activities of Glutathione-S-Transferase (GSH), Catalase (CAT), Superoxide Dismutase (SOD) were assayed in the liver tissue homogenate according to colorimetric method of Beutler et al. [29], Aebi [30] and Nishikimi et al. [31], respectively using Biodiagnostic kit according to the instruction of the supplier. Glutathione Reductase activity (GR) was assayed spectrophotometrically as described by Goldberg et al. [32] using Biodiagnostic kit according to the instruction of the supplier. Lipid peroxidation was also estimated in the liver tissue homogenate by measuring the value of Malondialdehyde (MDA) according to colorimetric method of Satoh [33] using Biodiagnostic kit according to the instruction of the supplier.
Physiological evaluation
The following biological parameters were estimated: total body weight, daily Body Weight Gain (BWG), percentage of Body Weight Gain (BWG%), Food Efficiency Ratio (FER), percentage of Food Efficiency Ratio (FER%) [34].
Histopathological investigations
A piece of pancreas and liver and one kidney were washed in sterile saline and fixed in 10% neutral formalin for histopathological studies. Tissues of liver, pancreas and kidney were dehydrated in gradual ethanol (50-99%), cleared in xylene, and embedded in paraffin. Microscopic sections were prepared and stained with haematoxylin and eosin (H and S) dye for microscopic investigation [35]. Olympus light microscope equipped with a digital camera was used in examination and photography of the stained sections.
Statistical analysis
Values were expressed as means ± the standard errors using SPSS program. The best group were chosen by analyzing data using ANOVA of SAS Package. The LSD (least significant difference was also computed using SAS.
Results
Blood glucose, hemoglobin A1c and α-amylase
Fasting blood glucose and hemoglobin A1c (Table 1) significantly (P<0.001) elevated after diabetes induction in the second diabetic group (G2) compared with the negative control group (G1). On the other hand, α-Amylase (Table 1) was nonsignificantly decreased as a result of diabetes in G2. Treating the diabetic rats of the third and fourth group with the aqueous extract of S. persica leaf significantly (P<0.001) decreased fasting blood glucose and hemoglobin A1c and restored them to that of the negative control group. Meanwhile, the level of α-Amylase non-significantly increased by treating the diabetic rats of G3 and G4 and nearly restored to the negative control values.
| Parameters | Statistics | G1 negative control |
G2 positive control |
G3 | G4 |
|---|---|---|---|---|---|
| FBG mg/dl | Mean ± SE | 87.33 ± 2.70b | 276.16 ± 1.47a | 99.66 ± 0.149c | 96.00 ± 0.085d |
| LSD 0.05=9.754 | |||||
| T-test | - | -57.38*** | 82.25*** | 50.45*** | |
| HbA1C% | Mean ± SE | 5.333 ± 0.176b | 7.933 ± 0.055a | 6.666 ± 0.149c | 6.200 ± 0.085d |
| LSD 0.05=0.3595 | |||||
| T-test | - | -13.11*** | 8.10*** | 15.97*** | |
| α-Amylase (u/l) | Mean ± SE | 45.00 ± 4.479a | 39.66 ± 2.654a | 43.16 ± 2.797a | 43.16 ± 2.785a |
| LSD 0.05=10.162 | |||||
| T-test | - | 0.86NS | -0.74NS | -1.12NS |
Data are represented as mean ± SE. T-test values; ***Significant at P<0.001. ANOVA analysis: within each row, means with different superscript; a,b,c,dSignificantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference; NSNon-Significant.
Table 1. Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on fasting blood glucose, hemoglobin A1c and α- Amylase.
Immunoglobulin’s (IgG, IgA and IgM)
Immunoglobulin’s (IgG, IgA and IgM) All immunoglobulin’s (IgG, IgA and IgM) indicate significantly (P<0.001) increased in the positive diabetic group (G2) compared with the negative control (Table 2). The cosupplementation of S. persica leaf aqueous extract in G3 and G4 significantly (P<0.001) decreased the studied immunoglobulin’s (IgG, IgA and IgM) and restored them to the normal values of that of the negative control.
| Immunoglobulin’s (mg/dl) | Statistics | G1 Negative control | G2 Positive control | G3 | G4 |
| IgG | Mean ± SE | 535.00 ± 3.01b | 744.33 ± 1.70a | 634.83 ± 5.71c | 601.50 ± 3.91d |
| LSD 0.05=20.724 | |||||
| T-test | - | -55.59*** | 18.85*** | 33.33*** | |
| IgM | Mean ± SE | 125.83 ± 2.24e | 359.33 ± 4.37a | 260.33 ± 3.92c | 245.33 ± 2.01d |
| LSD 0.05=9.523 | |||||
| T-test | - | -36.38*** | 16.26*** | 29.24*** | |
| IgA | Mean ± SE | 103.50 ± 3.64b | 343.66 ± 6.13a | 233.00 ± 3.92d | 304.16 ± 1.24c |
| LSD 0.05=10.850 | |||||
| T-test | - | -28.14*** | 21.91*** | 21.62*** | |
| IgE | Mean ± SE | 15.56 ± 0.57a | 14.06 ± 0.44b | 14.91 ± 0.58a | 14.90 ± 0.51a |
| LSD 0.05=1.769 | |||||
| T-test | - | 6.22*** | -1.16 NS | -0.90 NS |
Data are represented as mean ± SE; T-test values; ***Significant at P<0.001. ANOVA analysis: within each row, means with different superscript. a,b,c,dSignificantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference; NSNon-Significant.
Table 2. Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on serum immunoglobulin’s (IgG, IgM, IgA and IgE).
Liver enzymes
Liver function enzymes; ALT, AST, ALP and GGT activities significantly (P<0.001) increased in the positive diabetic group (G2) compared with the negative control (Table 3). The cosupplementation of S. persica leaf aqueous extract in G3 and G4 significantly (P<0.001) decreased the elevated liver function enzyme activity and restored them to the normal values of that of the negative control.
| Liver enzymes (U/l) | Statistics | G1 negative control | G2 positive control | G3 | G4 |
| ALT | Mean ± SE | 23.83 ± 2.02a | 23.00 ± 2.43a | 27.66 ± 2.92a | 22.66 ± 1.81a |
| LSD 0.05=7.237 | |||||
| T-test | - | 0.26 NS | -2.34* | 0.09 NS | |
| AST | Mean ± SE | 21.16 ± 0.70a | 21.00 ± 1.98a | 23.50 ± 2.07a | 21.83 ± 2.10a |
| LSD 0.05=4.885 | |||||
| T-test | - | 0.06NS | -1.09NS | -0.29NS | |
| ALP | Mean ± SE | 162.16 ± 10.08b | 186.50 ± 10.98a | 146.83 ± 4.71bc | 123.50 ± 5.14c |
| LSD 0.05=21.612 | |||||
| T-test | - | -5.15*** | 5.42*** | 4.06** | |
| GGT | Mean ± SE | 25.83 ± 2.22b | 22.33 ± 1.56b | 37.50 ± 2.75a | 24.50 ± 2.06b |
| LSD 0.05=6.278 | |||||
| T-test | - | 1.03NS | -3.85** | -0.81NS |
Data are represented as mean ± SE. T-test values; ***Significant at P<0.001. ANOVA analysis: within each row, means with different superscript; a,b,c,dSignificantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference; NSNon-Significant.
Table 3. Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on serum liver enzymes.
Bilirubin
Serum bilirubin (Table 4) was not significantly affected either by diabetes induction or treatment with S. persica leaf aqueous extract.
| Parameters (mg/dl) | Statistics | G1 negative control | G2 positive control | G3 | G4 |
| Total bilirubin | Mean ± SE | 0.566 ± 0.049a | 0.566 ± 0.033a | 0.600 ± 0.036a | 0.700 ± 0.073a |
| LSD 0.05=0.149 | |||||
| T-test | - | 0.00NS | -0.54NS | -1.86NS | |
| Direct bilirubin | Mean ± SE | 0.150 ± 0.022b | 0.183 ± 0.016ab | 0.166 ± 0.021ab | 0.233 ± 0.021ab |
| LSD 0.05=0.065 | |||||
| T-test | - | -1.00NS | 0.54NS | -1.46NS | |
| Indirect bilirubin | Mean ± SE | 0.416 ± 0.040a | 0.383 ± 0.040a | 0.433 ± 0.049a | 0.466 ± 0.071a |
| LSD 0.05=0.164 | |||||
| T-test | - | 0.59NS | -0.59NS | -1.11NS |
Data are represented as mean ± SE. ANOVA analysis: within each row, means with different superscrip; a,bsignificantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference. NSNon-Significant.
Table 4. Effect of Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on serum bilirubin.
Kidney function
Table 5 shows the kidney function parameters; urea, creatinine and uric acid. The positive control group (G2) showed significant (P<0.001) increase of all studied kidney function parameters compared with the negative control group. The co-supplementation of S. persica leaf aqueous extract in G3 and G4 significantly (P<0.001) decreased the all elevated kidney function parameters and restored them nearly to the normal values of that f the negative control. In addition, sodium and calcium ions were not significantly affected either by diabetes induction or treatment with S. persica leaf aqueous extract.
| Parameters mg/dl | Statistics | G1 negative control | G2 positive control | G3 | G4 |
| Urea | Mean ± SE | 22.33 ± 1.49c | 49.83 ± 1.42a | 26.78 ± 4.64b | 25.66 ± 1.58c |
| LSD 0.05=4.105 | |||||
| T-test | - | -10.61*** | 4.12*** | 11.94*** | |
| Creatinine | Mean ± SE | 0.600 ± 0.05d | 1.550 ± 0.056a | 0.833 ± 0.033bc | 0.633 ± 0.042d |
| LSD 0.05=0.128 | |||||
| T-test | - | -9.58*** | 23.32*** | 15.25*** | |
| Uric acid | Mean ± SE | 4.066 ± 0.223a | 3.833 ± 0.180ab | 3.850 ± 0.133ab | 3.416 ± 0.188b |
| LSD 0.05=0.508 | |||||
| T-test | - | 0.59NS | -0.07NS | 1.68NS | |
| Na+ | Mean ± SE | 131.33 ± 2.95a | 137.50 ± 1.25a | 138.33 ± 2.71a | 136.00 ± 2.03a |
| LSD 0.05=6.910 | |||||
| T-test | - | -1.72NS | -0.37NS | 0.57NS | |
| Ca++ | Mean ± SE | 9.233 ± 0.13b | 9.416 ± 0.21ab | 9.483 ± 0.13ab | 9.733 ± 0.10a |
| LSD 0.05=0.414 | |||||
| T-test | - | -1.10NS | -0.40NS | -1.49NS |
Data are represented as mean ± SE. T-test values; ***significant at P<0.001 ANOVA analysis: within each row, means with different superscript; a,b,c,dsignificantly different at P<0.05 whereas means superscripts with the same letters mean that there is no significant difference at P<0.05; LSD: Least Significant Difference; NSNon-Significant .
Table 5. Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on kidney function and electrolytes.
Serum lipids
Table 6 shows that induction of diabetes in G2 significantly (P<0.001) increased the level of Triglyceride (TG), Total Cholesterol (TC), Low Density Lipoprotein (LDL) and Very Low Density Lipoprotein (VLDL) and significantly (P<0.001) decreased the useful High Density Lipoprotein (HDL) compared with the negative control group. Treating diabetic rats in G3 and G4 with S. persica leaf aqueous extract significantly (P<0.001) decreased all elevated lipid profile (TG, TC, LDL and VLDL) and significantly (P<0.001) increased HDL and restored all lipid profile indices nearly to their normal values of the negative control.
| Serum lipids | Statistics | G1 negative control | G2 positive control | G3 | G4 |
|---|---|---|---|---|---|
| TG (mg/dl) | Mean ± SE | 99.16 ± 1.01b | 272.83 ± 1.44a | 205.00 ± 1.57c | 186.00 ± 1.48d |
| LSD 0.05=13.074 | |||||
| T-test | - | 83.0*** | 30.45*** | 56.51*** | |
| TC (mg%) | Mean ± SE | 132.50 ± 2.17c | 293.66 ± 1.74a | 223.00 ± 18.66b | 230.66 ± 1.38b |
| LSD 0.05=31.506 | |||||
| T-test | - | 45.84*** | 3.6*** | 24.04*** | |
| HDL (mg/dl) | Mean ± SE | 51.16 ± 1.47a | 25.83 ± 0.83b | 29.50 ± 0.99c | 32.66 ± 0.71d |
| LSD 0.05=2.179 | |||||
| T-test | - | 17.43*** | 3.2** | 41.0*** | |
| LDL (mg/dl) | Mean ± SE | 63.13 ± 2.88b | 210.46 ± 1.93a | 170.73 ± 1.91c | 159.00 ± 1.34d |
| LSD 0.05=9.754 | |||||
| T-test | - | ***-31.02 | ***13.59 | ***21.89 | |
| VLDL (mg/dl) | Mean ± SE | 19.83 ± 0.20b | 54.56 ± 0.28a | 41.00 ± 0.31c | 37.20 ± 0.29d |
| LSD 0.05=5.614 | |||||
| T-test | - | 83.0*** | 30.45*** | 56.51*** |
Data are represented as mean ± SE. T-test values; ***Significant at P<0.001, **significant at P<0.01. ANOVA analysis: within each row, means with different superscript; a,b,c,dsignificantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference.
Table 6. Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on serum lipids.
Antioxidant and lipid peroxidation
Table 7 shows that induction of diabetes in G2 significantly (P<0.001) decreased the activity of catalase, super oxide dismutase, glutathione-S-transferase and significantly (P<0.001) increased lipid oxidation (as determined by malondialdehyde) in the liver tissue homogenate compared with the negative control group. Treating diabetic rats in G3 and G4 with S. persica leaf aqueous extract significantly (P<0.001) increased all antioxidants and decreased MDA and restored them nearly to their normal values of the negative control.
| Antioxidant and MDA | Statistics | G1 Negative control | G2 Positive control | G3 | G4 |
| CAT (U/g) | Mean ± SE | 4.018 ± 0.048a | 0.418 ± 0.006f | 1.871 ± 0.041c | 2.580 ± 0.160b |
| LSD 0.05=0.209 | |||||
| T-test | - | 77.22*** | -38.78*** | -13.21*** | |
| SOD (U/g) | Mean ± SE | 1116.21 ± 6.59a | 422.88 ± 3.49f | 786.49 ± 4.01c | 814.01 ± 3.16b |
| LSD 0.05=18.285 | |||||
| T-test | - | 7.29*** | -370.66*** | -63.74*** | |
| GST (U/g) | Mean ± SE | 7727.66 ± 28.19a | 3172.50 ± 21.06f | 5113.33 ± 14.78c | 5487.16 ± 22.60b |
| LSD 0.05=74.858 | |||||
| T-test | - | 102.38*** | -81.62*** | -93.68*** | |
| Lipid peroxide (MDA) (nmol/g) liver tissue | Mean ± SE | 2.861 ± 0.152f | 11.883 ± 0.319a | 7.683 ± 0.064d | 6.968 ± 0.182e |
| LSD 0.05=0.532 | |||||
| T-test | - | -20.13*** | 13.59*** | 11.74*** |
Data are represented as mean ± SE. T-test values; ***Significant at P<0.001. ANOVA analysis: within each row, means with different superscript; a,b,c,dSignificantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05; LSD: Least Significant Difference.
Table 7. Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on antioxidants and lipid peroxidation in the kidney tissue homogenate of diabetic rats.
Physiological evaluation
Table 8 shows that induction of diabetes in G2 did not affect daily food intake and significantly (P <0.001) decreased Body Weight Gain (BWG), Body Weight Gain percentage (BWG%), Food Efficiency Ratio (FER) and Food Efficiency Ratio percentage (FER%) compared with the negative control group. Treating diabetic rats in G3 and G4 with S. persica leaf aqueous extract significantly (P<0.001) increased BWG, BWG %, FER and FER% and restored them nearly to their normal values of the negative control.
| Physiological evaluation | Statistics | G1 Negative control | G2 Positive control | G3 | G4 |
| FI (g/d) | Mean ± SE | 16.433 ± 0.316a | 16.233 ± 0.252a | 16.43 ± 0.228a | 16.46 ± 0.218a |
| LSD 0.05=0.418 | |||||
| T-test | - | 1.36NS | -1.06NS | -1.22NS | |
| BWG (g/4 week) | Mean ± SE | 34.833 ± 2.329a | -7.833 ± 1.013b | 27.66 ± 0.843a | 28.16 ± 1.013a |
| LSD 0.05=8.191 | |||||
| T-test | - | 16.55*** | -57.33*** | -20.33*** | |
| BWG (g/d) | Mean ± SE | 1.161 ± 0.077a | -0.261 ± 0.033c | 0.922 ± 0.028b | 0.938 ± 0.033b |
| LSD 0.05=0.171 | |||||
| T-test | - | 16.57*** | -57.45*** | -20.35*** | |
| BWG (%) | Mean ± SE | 23.580 ± 1.793a | -5.291 ± 0.653c | 15.16 ± 0.537b | 15.44 ± 0.624b |
| LSD 0.05=3.933 | |||||
| T-test | - | 14.54*** | -45.32*** | -18.52*** | |
| FER (g/d) | Mean ± SE | 0.070 ± 0.004a | -0.016 ± 0.002b | 0.056 ± 0.001a | 0.057 ± 0.002a |
| LSD 0.05=0.021 | |||||
| T-test | - | 16.54*** | -54.29*** | -19.79*** | |
| FER (%) | Mean ± SE | 7.065 ± 0.472a | -1.608 ± 0.208c | 5.612 ± 0.171b | 5.702 ± 0.205b |
| LSD 0.05=1.049 | |||||
| T-test | - | 16.55*** | -54.29*** | -20.26*** |
Data are represented as mean ± SE. T-test values; ***Significant at P<0.001. ANOVA analysis: within each row, means with different superscript; a,b,c,dSignificantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference; NSNon-Significant.
Table 8. Effect of treating alloxan induced diabetic rats with S. persica leaf aqueous extract on physiological evaluation.
Histopathology
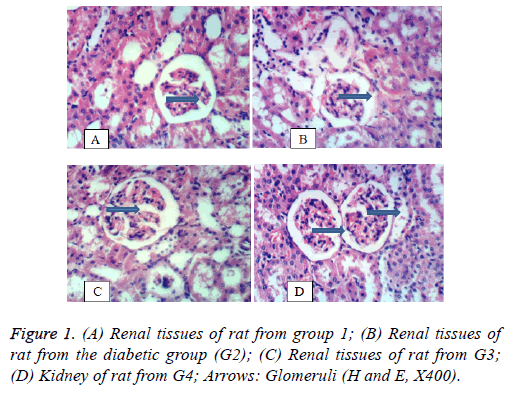
Kidney: Figure 1 shows the histology of kidney. Figure 1A shows renal tissues of a rat from the negative control group (G1) showing normal histological structure of renal parenchyma. Figure 1B shows renal tissues of a rat from the diabetic positive control group (G2) with vacuolation of epithelial lining renal tubules and atrophy of glomerular tuft. Figure 1C shows renal tissues of a rat from group 3 treated with the lowest concentration of S. persica showing slight vacuolation of epithelial lining renal tubules and atrophy of glomerular tuft. Figure 1D shows kidney of rat from group 4 treated with the highest concentration of S. persica with restored normal renal tissues showing no histopathological changes.
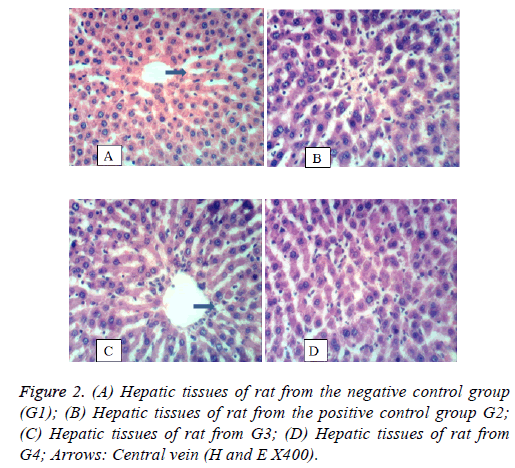
Liver: Figure 2 shows the histology of liver. In Figure 2A, hepatic tissues of rat from group 1 showing normal histological structure of hepatic lobule. In Figure 2B, hepatic tissues of rat from the diabetic untreated group (G2) showing focal hepatic necrosis associated with inflammatory cells infiltration. Figure 2C shows the hepatic tissue of a rat from G3 showing nearly normal tissues as a result of treatment with S. persica. Figure 2D, hepatic tissues of a rat from group 4 showing restored normal hepatic tissues.
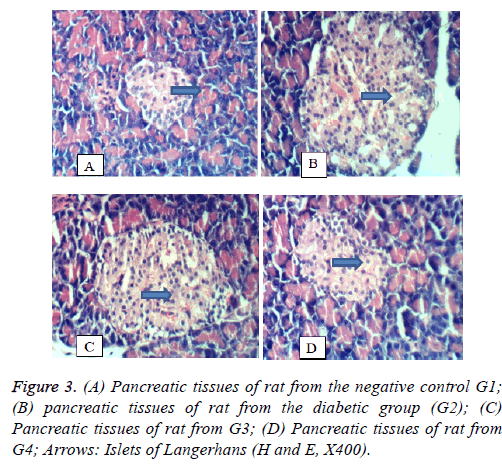
Pancreas: Figure 3 shows the histology of pancreas in the 4 groups under study. In Figure 3A, the pancreatic tissues of the negative control rats (G1) showed no histopathological changes. In Figure 3B, the pancreatic tissues of rat from the diabetic group (the positive control group, G2) showing histopathological changes illustrated in vacuolation of islets of Langerhan’s. In Figure 3C, the pancreatic tissues of rat from G3 showing slight vacuolation of islets of Langerhan’s. Figure 3D, pancreatic tissues of rat from group 4 showing no histopathological changes.
Discussion
This study was focused at studying the medicinal benefit of S. persica leaf aqueous extract. To achieve the objective of this study, diabetes was induced in male rats by the intraperitoneal injection of alloxan according to Dash et al. [12] and two doses of leaf aqueous extract were used in treating the alloxan diabetic rats for 28 d. In this study, induction of diabetes damaged insulin secreting β-pancreatic cells, reduces the endogenous insulin release and lowers the intake of glucose by the cells which leads to increase blood sugar level and hemoglobin A1c [36-38]. The serum alpha-amylase which is considered as an indication of proper pancreatic function [39,40] was also decreased as a result of diabetes and nearly restored its normal levels by treating with S. persica leaf aqueous extract. In addition, the immunoglobulin’s (IgG, IgA and IgM) levels were increased by diabetes [38,41] and they were decreased with the administration of the aqueous extract of S. persica to the alloxan induced diabetic rats in G3 and G4 for 4 weeks. This result is consistent with recent studies by Deepti et al. [42] who reported that there was a positive correlation between the two parameters elevated HbA1c and Immunoglobulin level. Immunoglobulin levels in diabetes revealed significant increase of IgG, IgA and IgM in patients compared to healthy controls [9,43].
In the current study, serum liver enzymes; AST, ALP, GGT and ALT were elevated as a result of alloxan induced diabetes reflecting active liver damage and inflammatory hepatocellular disorders result in extremely elevated transaminase levels [44,45]. Therefore, the increment of the activities of AST, ALT and ALP in plasma may be mainly due to the leakage of these enzymes from the liver cytosol into the blood stream [46]. Treating the diabetic rats with S. persica leaf aqueous extract nearly restored all liver enzymes to their normal levels [9,43].
In addition, the increased level of serum protein, albumin and globulin in alloxan induced diabetic rats are presumed to be due to the increased protein catabolism and gluconeogenesis during diabetes [47].
The elevation in renal parameters caused by alloxan induced diabetes in the positive control diabetic group is a sign of the major complication of diabetes called diabetic nephropathy [9,38,43]. On the other hand, treating the diabetic rats with S. persica leaf aqueous extract succeeded in restoring all altered renal parameters nearly to the negative control value [43].
Alloxan induced hyperglycemia in the current study decreased all antioxidants and increased lipid peroxidation due to the increased oxidative stress and free radicals. This is ascribed to the increase in oxidative stress and depleting the activity of the anti-oxidative defense system and resulting in elevated levels of oxygen free radicals [48]. Treating the diabetic rats with S. persica leaf aqueous extract succeeded in increasing all antioxidant and decreasing lipid peroxidation restoring them to the negative control values [43].
In the current study, diabetic elevated the levels of serum TC, TG, LDL-C and VLDL-C and decreased the level of HDLC compared with normal control rats [49]. Treating the diabetic rats with the two doses of S. persica leaf aqueous extract succeeded in lowering TC, TG, LDL-C and VLDL-C and increased the level of HDLC. This result is consistent with that of El-Gawad [50] who reported that alloxan treatment reduced the body weight gain of rats and increased the organ-to-body weight ratio for liver, kidney and pancreas.
Conclusion
The current study revealed that S. persica leaf aqueous extract succeeded in controlling hyperglycemia. In addition, S. persica leaf aqueous extract revealed also an antioxidant and hypolipidemic activity in alloxan induced diabetic male rats. It restored all biochemical parameters and the injured kidney, liver and pancreas tissues nearly to the normal as in the negative control group.
Conflict of Interest
The authors have no conflict of interests.
References
- Ahmad H, Rajagopal K. Salvadora persica L. (Meswak) in dental hygiene. Saudi J Dent Res 2014; 5: 130-134.
- Trovato A, Galati EM, Rossitto A, Monforte MT, d'Aquino A, Forestieri AM. Hypoglycemic effects of Salvadora persica L. in the rat. Phytomedicine 1998; 5: 129-132.
- Khatak M, Khatak S, Siddqui AA, Vasudeva N, Aggarwal A, Aggarwal P. Salvadora persica. Pharmacogn Rev 2010; 4: 209-214.
- Saini S, Yadav JP. Antidiabetic and antihyperlipidemic effects of ethanolic extract of Salvadora persica L. on alloxan-induced diabetic rats. Der Pharmacia Sinica 2013; 4: 178-182.
- Hooda MS, Pal R, Bhandari A, Singh J. Antihyperglycemic and antihyperlipidemic effects of Salvadora persica in streptozotocin-induced diabetic rats. Pharm Biol 2014; 52: 745-749.
- Khan M, Ali M, Ali A, Mir SR. Hypoglycemic and hypolipidemic activities of Arabic and Indian origin Salvadora persica root extract on diabetic rats with histopathology of their pancreas. Int J Health Sci Qassim Univ 2014; 8: 45-56.
- Almas K, Skaug N, Ahmad I. In vitro antimicrobial comparison of miswak extract with commercially available non-alcohol mouthrinses. Int J Dent Hyg 2005; 3: 18-24.
- Al-Sieni AI. The antibacterial activity of traditionally used Salvadora Persica L. (Miswak) and Commiphora Gileadensis (Palsam) in Saudi Arabia. Afr J Tradit Complement Altern Med 2014; 11: 23-27.
- El Rabey HA, Almutairi FM, Al-Sieni AI; Al-Seeni MN, Al-Duais MA, Sakran MI, Elbakry MA. The antioxidant, antidiabetic and antilipidemic activity of Salvadora persica twig in alloxan diabetic male rats. IJBB 2017; 54: 314-322.
- Knapka JJ, Judge FJ. The effects of various levels of dietary fat and apple supplements on growth of golden hamster. Lab Anim Sci 1974; 24: 318-325.
- Sharma D, Dey YN, Sikarwar I,Sijoria R, Wanjari MM, Jadhav AD. In vitro study of aqueous leaf extract of Chenopodium album for inhibition of calcium oxalate and brushite crystallization. Egyptian J Basic Appl Sci 2016; 3: 164-171.
- Dash GK, Suresh P, Ganapaty S. Studies on hypoglycaemic and wound healing activities of Lantana camara Linn. J Natl Remedies 2001; 1: 105-110.
- Barham D, Trinder P. GOD-PAP enzymatic colorimetric method of glucose estimation without deproteinization. Analyst 1972; 97: 312-322.
- Variant. Hemoglobin Aic program. Instruction manual. Bio Red Dignostic Group, USA 1997; 1-20.
- Ying Foo A, Bais R. Amylase measurement with 2-chloro-4-nitrophenyl maltotrioside as substrate. Clin Acta 1998; 272: 137-147.
- Berne G. Detection of total IgG. Clin Chem 1974; 200: 61-89.
- Thefeld W, Hffmiester H, Busch EW, Koller PU, Volmer J. Reference value for determination of GOT (glutamic opal acetic transaminase), GPT (glutamic pyruvic transaminase) and alkaline phosphatase in serum with optimal standard methods. Deut Med Wochenchr 1974; 99: 343-351.
- Schlebusch H, Rick W, Lang H, Knedel M. Standards in the activities of clinically important enzymes. Dtsch Med Wochenschr 1974; 99: 765-766.
- Gowenlock AH, McMurray JR, Mclauchlan DM. Varley's pratical clinical biochemistery (6th edition). CBC Publishers and Distributors 1988.
- Fawcett J, Scott J. A rapid and precise method for the determination of urea. J Clin Pathol 1960; 13: 156-159.
- Fossati P, Prencipe L, Berti G. Enzymatic colrorimetric method of determination of uric acid in serum and urine. Clin Chem 1980; 26: 227-231.
- Tietz NW. Fundamentals of clinical chemistry. W.B. Saunders 1976; 1211.
- Schoenfeld RG, Lewellen C. A colourimetric method for determination of serum chloride, Clin Chem 1964; 10: 533.
- Morin LG. Direct colorimetric determination of serum calcium with o-cresolphthalein complexon. Am J Clin Pathol 1974; 61: 114-117.
- Balistreri WF, Shaw LM. Liver function. Fundamentals of clinical chemistry (3rd ed). WB Saunders, Philadelphia 1987; 729-761.
- Young DS. Effects of drugs on clinical laboratory tests (4th ed). AACC Press, Washington 1995.
- Naito HK. High-density lipoprotein (HDL) cholesterol. Clin Chem 1984; 437: 1207-1213.
- Srivastava LM, Das N, Sinha S. Essentials of practical biochemistry. CBC Publishers and Distributers, India 2002.
- Beutler E, Duron O, Kelly MB. Improved method for the determination of blood glutathione. J Lab Clinic Med 1963; 61: 882-890.
- Aebi H. Catalase in vitro. Methods Enzymol 1984; 105: 121-126.
- Nishikimi M, Roa NA, Yogi K. Measurement of superoxide dismutase. Biochem Bioph Res Common 1972; 46: 849-854.
- Goldberg DM, Spooner RJ. Assay of glutathione reductase (3rd ed). Verlag Chemie Deerfield Beach 1983; 3: 258-265.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 1978; 90: 37-43.
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res 1993; 10: 1093-1095.
- Drury R, Wallington E, Cancerson R. Carleton's histological technique (4th ed). Oxford Univ Press, Oxford, UK 1976.
- Lenzen S. The mechanism of alloxan and streptozotocin-induced diabetes. Diabetologia 2008; 51: 216-226.
- Matheka DM, Kitua M, Alkizim F. Peculiar glycemic patterns in alloxan induced diabetes animal model. Afr J Pharm Ther 2012; 1: 30-34.
- Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa Oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. BioMed Res Int 2015; 381040.
- Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci 2007; 52: 1-17.
- Sales PM, Souza PM, Simeoni LA, Magalhães PO, Silveira D. α-Amylase Inhibitors: A review of raw material and isolated compounds from plant source. J Pharm Pharmaceut Sci 2012; 15: 141-183.
- Qusti S, El Rabey HA, Balashram S. The hypoglycemic and antioxidant activity of cress seed and cinnamon on streptozotocin induced diabetes in male rats. Evid Based Complement Altern Med 2016; 5614564.
- Deepti C, Reena K, Pooja S, Gopinath V, Rohit A, Vijayta A. Co-relating HbA1c and serum IgA in diabetic and non-diabetic patients with and without periodontitis. Asian J Med Sci 2015; 6: 72-77.
- El Rabey HA, Al-Seeni MN, Bakhashwain A. The Antidiabetic activity of nigella sativa and propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Evid Based Complement Alternat Med 2017: 5439645.
- Hultcrantz R, Glaumann HL, Lindberg G. Liver investigation in 149 asymptomatic patients with moderately elev