- Biomedical Research (2011) Volume 22, Issue 3
Salivary Total Protein, Sialic Acid, Lipid Peroxidation and Glutathione in Oral Squamous Cell Carcinoma
Shivashankara AR and Kavya Prabhu M
Department of Biochemistry, Father Muller Medical College, Mangalore, Karnataka, India.
Accepted Date: March 29 2011
Abstract
Saliva as a diagnostic tool, is gaining attention world wide. Due to non-invasiveness of its collection and non-necessity of skilled technician for collection, and presence of sensitive biomarkers in saliva, it is considered as a worthwile diagnostic tool. Present study made an attempt to evaluate the status of proteins, sialic acid and oxidative stress in oral squamous cell carcinoma. Unstimulated whole saliva samples were collected from healthy controls and oral squamous cell carcinoma (OSCC) patients. Salivary levels of total proteins, free sialic acid, protein-bound sialic acid, malondialdehyde and glutathione were assayed by spectro-photometric methods. OSCC patients had higher salivary levels of total proteins, free sialic acid, protein-bound sialic acid and malondialdehyde, and lower levels of glutathione, when compared to healthy controls. The changes correlated with progression of cancer as evident by more pronounced changes of above biochemical parameters in well-differentiated cases of OSCC, compared to moderately differentiated OSCC and patients with pre-malignant le-sions of OSCC. Elevated levels of proteins and sialic acid suggest role of glycoproteins in carcinogenesis. Oxidative stress is involved in etiopathology of oral cancer, as evident from elevated malondialdehyde and decreased glutathione. Salivary parameters could be of use in diagno-sis and prognosis of oral cancer.
Keywords
Antioxidants, Oral squamous cell carcinoma, Glutathione, Malondialdehyde, Oxidative stress, Sialic acid.
Introduction
Cancer is the second leading cause of death worldwide. Eleven million new cases of cancer are diagnosed every year [1]. It is estimated that there are approximately 2-2.5 million cases of cancer in India at any given point of time with around 7,00,000 new cases being detected every year . Head and neck squamous cell carcinoma occurs in 50,000 new cases annually in the USA, results in more than 13,000 deaths every year [2]. India has the highest incidence of oral squamous cell carcinoma in the world [3,4]. About 50-70% of cancer-related deaths in India are due to oral cancer. [1,5]. Age-standardised incidence rates of oral cancer per 100,000 population in India were estimated to be 12.8 in men and 7.5 in women [3,4]. The data from WHO Global Oral Health Programme sug-gested that tobacco use and excessive alcohol consump-tion were estimated to account for about 90% of oral can-cers [3-5]. Hereditory factors and viral infections, along with lifestyle and dietary factors contribute to the etiology of oral cancer [6].
Glycoconjugates play important role in malignant trans-formation of cells. Aberrant glycosylations are the uni-versal phenomena in malignant transformation of cells. Carbohydrate residues of glycoproteins and glycolipids are released into the circulation through increased turn-over, secretion and/ or shedding , and are of immense in-terest for their diagnostic and prognostic value in cancer [7-9]. Increased serum levels of total proteins (TP), total sialic acid (TSA), lipid-bound sialic acid (LSA), and in-creased ratio of TSA to TP, have been reported by previ-ous works. Studies have indicated the usefulness of blood levels of TSA, LSA and TSA to TP ratio in diagnosis, prognosis and management of cancers of various types [7-9].
Free radicals are implicated in the pathogenesis of multi-stage process of carcinogenesis. They are proposed to cause DNA base alterations, strand breaks, damage to tumor suppressor genes and enhanced expression of proto-oncogenes [10-13]. The burst of reactive oxygen species and reactive nitrogen species, has been implicated in the development of cancer. Increased blood levels of lipid hydroperoxide, malondialdehyde, and decreased blood levels of antioxidants catalase, superoxide dismu-tase, glutathione peroxidase, vitamin C and vitamin E, have been reported in oral cancer patients [13-16].
Saliva as a diagnostic tool, has gained lot of attention in last three decades [17,18]. Whole saliva can be collected non-invasively and no special equipment or skilled person is needed for saliva collection. Salivary chemical consti-tuents have been analyzed in autoimmune diseases, syste-mic diseases, cancer and infectious conditions. Salivary levels of hormones and drugs were also monitored [17-23]. In India, few researchers have attempted salivary analysis in cancer, oral diseases and infections [24-26].
Salivary analysis is not given due thrust in India. There is paucity of studies on analysis of salivary glycoconjugates and oxidative stress markers in cancer. Because of its proximity to oral neoplasms and pre-malignant lesions, saliva could be the ideal tool for screening, diagnosis, and management of oral cancer.Present study has made an attempt to evaluate the status of salivary total proteins, sialic acid, malondialdehyde and glutathione in oral squamous cell carcinoma.
Materials and Methods
Source of Data
The study was hospital-based and was carried out at Fa-ther Muller Medical College and Hospital, Mangalore. The study protocol was approved by the Ethical Commit-tee of the institution. Following were the study groups :
Group I : Controls- apparently healthy male volunteers in the age group of 20-60 years, n = 50;
Group –II : Comprised of patients who visited the oncol-ogy department and diagnosed of oral squamous cell car-cinoma. Only male patients were included. The diagnosis of OSCC was based on clinical examination and histopa-thological study. The subjects of group-II were further subdivided into three subgroups :
A)
Patients with pre-malignant lesions of OSCC
(leukoplakia, fibrosis),
B)
Moderately differentiated cases of OSCC,
C)
Well-differentiated cases of OSCC.
Total number of subjects in group-II was seventy. In-formed consent was obtained from all the subjects of the study.
Detailed history of habits (smoking, alcohol abuse, to-bacco chewing), chronic illness and clinical history, was collected from the subjects.
Exclusion criteria : Chronic smokers, alcoholics, tobacco chewers and those with any chronic illness or systemic diseases, were excluded from group-I. Patients with any chronic illness or systemic diseases and cancers other than oral cancer, were excluded from group-II.
Sample collection
Unstimulated saliva samples were collected between 9-11 AM, according to the method of Navazesh (27). This was to ensure that the variability in salivary flow rate and composition, be minimized. The subject was asked to rinse the mouth with distilled water thoroughly to remove any food debris and then after 10 minutes, directed to spit into a sterile plastic container. Forcible spitting was avoided.
Analysis of Saliva
The saliva samples were centrifuged at 3000 rpm for 10 minutes and the supernatant was taken for analysis of fol-lowing parameters:
1.
Total Proteins : estimated by Lowry’s method (28),
2.
Sialic acid : Saliva was treated with ethanol to pre-cipitate proteins. Sialic acid contents of both pre-cipitate and supernatant, were estimated. This was based on reaction of sialic acid with acid ninhydrin reagent. The absorbance of colored product was measured at 470nm. Sialic acid contents of super-natant and precipitate were considered as “ free sialic acid” and “protein-bound sialic acid” respec-tively (29),
3.
Malondialdehyde, the marker of lipid peroxidation, was estimated as thiobarbituric acid-reactive sub-stances (TBARS) (30),
4.
Glutathione, reduced (GSH) : GSH was estimated by the method of Beutler et al (31), which was based on reduction of 5,5/ -dithiobis-(2 nitrobenzoic acid) by GSH.
Statistical Analysis
The values were expressed as mean ±S.D. The data were analyzed by Student’s t test for significance of differ-ences between each group.
Results
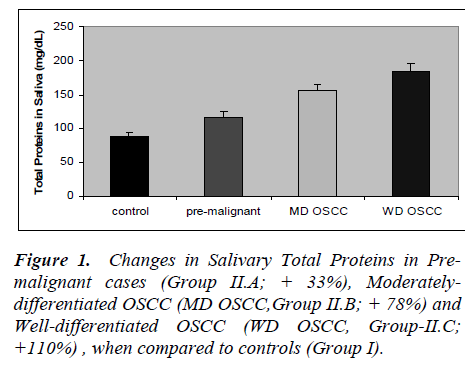
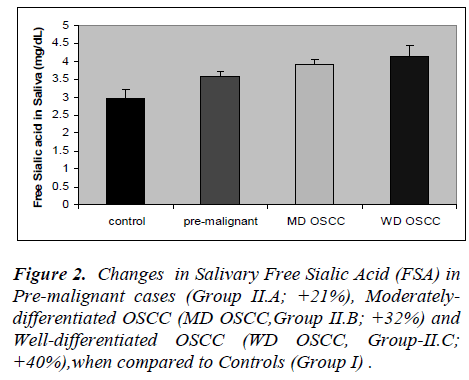
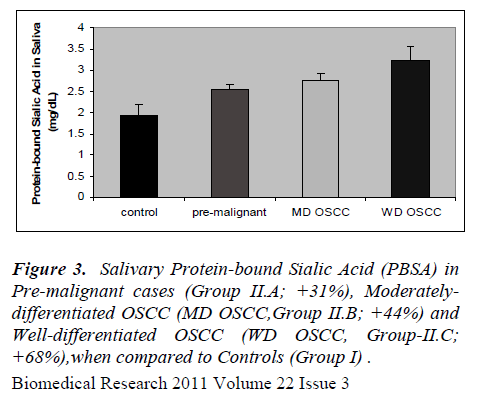
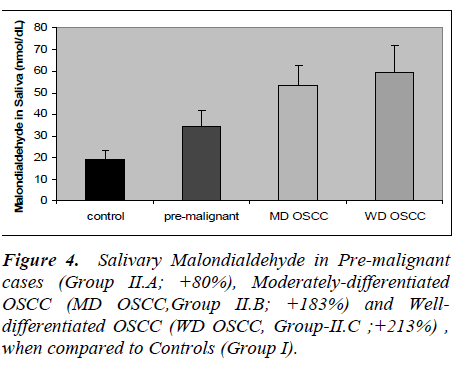
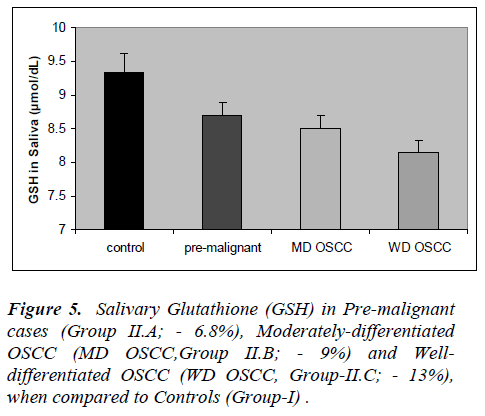
The results of the present study are presented in figures 1 to 5. Salivary levels of total proteins, free sialic acid, pro-tein-bound sialic acid and malondialdehyde were higher in OSCC cases than controls. Salivary malondialdehyde levels were higher and glutathione levels were lower in OSCC than controls. All the results were statistically sig- nificant (P values less than 0.001). Changes in all the pa-rameters correlated with progression of cancer as evident from more pronounced changes in well-differentiated cases of OSCC compared to moderately differentiated cases of OSCC and pre-malignant lesions. However, there was no statistically significant difference in malondialde-hyde values of well-differentiated OSCC and moderately differentiated OSCC; also no statistically significant dif-ference in GSH values of pre-malignant and moderately differentiated OSCC. Except these two, all the other re-sults were statistically significant (P < 0.001).
Discussion
Present study revealed altered salivary total protein and sialic acid status in OSCC. The changes were seen not only in well-differentiated cases but also in moderately differentiated OSCC and cases of pre-malignant lesions. Sialic acid is the major carbohydrate component of gly-coproteins , being present at their termini. Increased se-rum levels of total proteins (TP), total sialic acid (TSA), lipid-bound sialic acid (LSA), and increased ratio of TSA to TP, have been reported by previous studies, in cancers of cervix, ovary, breast, colon and oral cavity (7-9). Cir-culatory levels of sialic acid are proposed to reflect changes in cell surface characteristics, adhesiveness and cellular invasiveness. Salivary or serum levels of sialic acid altered also in non-cancerous conditions like diabetes mellitus, pregnancy and inflammations. Hence, workers in this field considered sialic acid as a sensitive but, not specific tumor marker [7-9]. Salivary proteins and sialic acid were assayed by few researchers in cases of OSCC. Sanjay et al [26] observed elevated levels of total pro-teins, free sialic acid and protein-bound sialic acid in the saliva in OSCC. Elevated salivary levels of proteins de-fensin-1 and statherin were seen in oral cancer [32,33]. Salivary levels of epidermal growth factor, CA-125, CA- 15-3 and CEA were evaluated as tumor markers in can-cers of oral cavity, breast, ovary and cervix [17,23]. San-jay et al (26) observed increased salivary level of total sugars in oral cancer. Some researchers have hinted at possible interactions between antioxidant enzymes and free sialic acid in saliva. They also postulated its role as a hydroxyl radical scavenger, as a component of salivary mucin [34].
Present study revealed increased malondialdehyde levels in oral cancer patients and in patients with pre-malignant lesions, indicating increased lipid peroxidation. In the present study, salivary GSH levels were significantly lower in cancer patients and those with pre-malignant lesions. Previous studies have reported increased blood levels of lipid peroxides and malondialdehyde, and de-creased blood levels of antioxidants in oral cancer [13-15]. Patel et al [13] showed risk of oral cancer develop-ment in tobacco users with lower antioxidant enzymes and higher lifetime tobacco exposure.
Saliva is equipped with enzymatic and non-enzymatic antioxidants. The salivary antioxidant system, is proposed to have a fundamental anticarcinogenic role in the oral cavity, and is aimed at fighting reactive oxygen spe-cies(ROS) and reactive nitrogen species(RNS), which originate from smoking, alcohol, food, drink, and/or vari-ous other volatile sources, entering freely into the oral cavity through the mouth. An age-related reduction in the salivary antioxidant capacity, has been reported (35). Almadori et al [36] reported higher salivary levels of GSH in patients of oral and pharyngeal cancers when compared to healthy controls and patients of laryngeal cancer. There has been a report of decreased levels of antioxidants, and increased levels of protein - and DNA-oxidation products in saliva of OSCC patients [37].
Glutathione (GSH) is the major thiol compound of human body with multiple functions. It is an inevitable molecule concerned with free radical scavenging and detoxifica-tion. Decreased GSH level in saliva in OSCC suggests its depletion due to increased oxidative stress. GSH-mediated action of glutathione S-transferase is responsi-ble for detoxification of many xenobiotics including car-cinogens (38). Increased utilization of GSH in detoxification of carcinogens thus, increased demand could be the reason for its decreased levels in oral cancer.
Conclusions
Saliva as a diagnostic tool has distinct advantage of non-invasiveness of sample collection and non-necessity of skilled persons for its collection. Salivary sialic acid and oxidative stress could serve as sensitive markers of oral squamous cell carcinoma. There is a need for detailed study with larger sample size, correlating findings of sali-vary analysis with those of blood analysis, before estab-lishing saliva as a reliable laboratory tool for clinical ap-plications.
Acknowledgements
We are indebted to Rev.Fr.Patrick Rodrigues, the director, Rev.Fr.Denis D’Sa, the administrator, Dr. Jayaprakash Alva, the dean and Dr.B.Sanjeev Rai, the chief of medical services, for the infrastructure and support. We thank Dr.Malathi M, Professor and Head of Biochemistry for her kind encouragement and co-operation. The authors are grateful to Indian Council of Medical Research, New Delhi, for the grant of a Short Term Research Fellowship to Ms.Kavya Prabhu, to carry out a part of this research project.References
- Park K. Park’s Text Book of Preventive and Social Medicine, 19th edition, M/s B. Bhanot; 2007: 313- 315.
- American Cancer Society. Cancer facts and figures-1997. American Cancer Society; 1997.
- National Cancer Registry Programme. Annual report. ICMR; 2005.
- WHO Oral Health Programme. Global data on inci- dence of oral cancer. WHO; 2005.
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. In: IARC Cancer Base no.5, version 2.0, IARC Press; 2004.
- Chong SC, Chandramouli GV, Saleh A, et al. Gene expression in human oral squamous cell carcinoma is influenced by risk factor exposure. Oral Oncol 2009; 45: 712-719.
- Baxi BR, Patel PS, Adhavaryu SG,Dayal PK. Useful- ness of serum glycoconjugates in precancerous and cancerous diseases of oral cavity. Cancer 1991; 67: 135-140.
- Raval GN, Parekh LJ, Patel DD, Jha FP, Sainger RN and Patel PS. Clinical usefulness of alterations in sialic acid, sialyl transferase and sialoproteins in breast can- cer. Indian J Clin Biochem 2004; 19: 60-71.
- Rajpura KB, Patel PS ,Chawda JG, Shah RM. Clinical significance of total and lipid-bound sialic acid levels in oral pre-cancerous conditions and oral cancer. J Oral Pathol Med 2005; 34: 263-267.
- Cerutti PA. Oxy-radicals and cancer. Lancett 1994; 344: 862-863.
- Gonalez RA. Free radicals, oxidative stress and DNA metabolism in human cancer. Cancer Invest 1999; 17: 376-377.
- 12 Irshad M, Chaudhuri PS. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol 2002; 40: 1213-1232.
- Patel BP, Rawal UM, Raval RM, Shukla SN, Patel PS. Tobacco, antioxidant enzymes, oxidative stress and ge- netic susceptibility in oral cancer. Am J Clin Oncol 2008; 31: 454-459.
- Sultan Beevi SS, Hassannal Rasheed AM, Geetha A. Evaluation of oxidative stress and nitric oxide levels in patients with oral cavity cancer. Jap J Clin Oncol 2004; 34: 379-385.
- Manoharan S, Kolanjappan K, Suresh K, Panjamurthy K. Lipid peroxidation and antioxidant status in patients with oral squamous cell carcinoma. Indian J Med Res 2005; 122: 529-534.
- Stich HF, Anders F. The involvement of reactive oxy- gen species in oral cancer of betelquid-tobacco chew- ers. Mutation Res 1989; 214: 47-61.
- Kaufman E, Lamster I B. The diagnostic applications of saliva, a review. Crit Rev oral Biol Med 2002; 13: 197-212.
- Ziang L, Xiao H, Wong DT. Salivary biomarkers for clinical application. Mol Diagn Ther 2009; 13: 245-259.
- SahebJamee M, Eslami M, AtarbashmiMoghadam F, Sarafnejad A. Salivary concentration of TNF alpha, IL 1 alpha, IL 6, and IL 8 in oral squamous cell carci- noma. Med Oral Patol Oral Cir Bucal 2008; 13: E292- 295.
- Streckfus CF, Bigler R, Navazesh M, Al-Hashimi I. Cytokine concentrations in stimulated whole saliva among patients with primary Sjogren’s syndrome, and primary Sjogren’s with secondary Sjogren’s syndrome, and primary Sjogren’s syndrome receiving varying doses of interferon for symptomatic treatment of the condition: a preliminary study. J Clin Oral Invest 2001; 5: 133-135.
- Groschl M. Current status of salivary hormone analy- sis. Clin Chem 2008; 54: 1759-1769.
- Streckfus C, Bigler L. The use of soluble, salivary c-erb B-2 for the detection and post-operative follow-up of breast cancer in women: the results of a five year trans- lational study. Adv Dent Res 2005; 18: 17-24.
- Nagler R, Bahar G, Shpitzer T, Feinmesser R. Con- comitant analysis of salivary tumor markers- a new di- agnostic tool for oral cancer. Clinical Cancer Research 2006; 12: 3979-3984.
- Shetty PK, Pattabiraman TN. Salivary glycoproteins as indicators of oral diseases. Indian J Clin Biochem 2004; 19: 97-101.
- Moorthy M, Daniel HD, Abraham P. An evaluation of saliva as an alternative to plasma for the detection of hepatitis C virus antibodies. Indian J Med Microbiol 2008; 26: 327-332.
- Sanjay PR, Hallikeri K, Shivashankara AR. Evaluation of salivary sialic acid, total protein and total sugar in oral cancer: A preliminary report. Indian J Dent Res 2008; 19 : 288-291.
- Navazesh M. Methods for collecting saliva. Ann Ny Acad Sci 1993; 20: 72-73.
- Lowry OH, Rosebrough NJ, Farr AL, Rabdall RJ. Pro- tein measurement with Folin’s phenol reagent.J Biol Chem 1951; 193:265-275.
- Yao K, Ubuka T, Masuoka N, Kinuta M, Ikeda T. Di- rect determination of bound sialic acids in sialoglyco- proteins by acidic ninhydrin. Anal Biochem 1989; 179: 332-335.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid perox- ides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963; 61: 882-888.
- Contucci AM, Inzitari R, Agostino S, Fiorita A, Cabras T, Scarano E, et al. Statherin levels in saliva of patients with precancerous and cancerous lesions of the oral cavity a preliminary report. Oral Oncology 2005; 11: 95-99.
- Mizukawa N, Sugiyama K, Fukunaya J, et al. Defensin- 1, a polypeptide detected in the saliva of oral squamous cell carcinoma patients. Anticancer Res 1998; 18: 4645-4649.
- Ogasawara Y, Namai T, Yoshino F, Lee Me, Ishii K. Sialic acid is an essential moiety of mucin as a hy- droxyl radical scavenger. FEBS Lett 2007; 58: 2473- 2477.
- Hershkovich O, Shafat I, Nagler RM. Age-related changes in salivary antioxidant profile : possible impli- cations for oral cancer. J Gerontol A Biol Sci Med Sci 2007; 62: 361-366.
- Almadori G, Bussu F, Galli J, Limongelli A, Persichilli S, Zappacoasta B, Minucci A, Paludetti G, Giardina B. Salivary glutathione and uric acid levels in patients with head and neck squamous cell carcinoma. Head and Neck 2007; 29 : 648-654.
- Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM. Salivary analysis in oral cancer patients : DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer 2007; 109: 54-59.
- Rana SVS, Allen T, Singh R. Inevitable glutathione, then and now. Indian J Exp Biol 2002; 40: 706-716.