- Biomedical Research (2011) Volume 22, Issue 1
Saliva C- reactive protein levels in patients with acute urticaria
Nivedita L. Rao1*, Sukanya Shetty2, Jyothi D’Souza1, Prasad R. M3, Vishal B4, Shariff M. H5 and Raghavendra U11Department of Biochemistry, Yenepoya Medical College, Deralakatte, Mangalore, India
2Department of Biochemistry, K. S. Hegde Medical Academy, Deralakatte, Mangalore, India
3Department of Biochemistry, A. J. Institute of Medical Sciences, Mangalore, India
4Department of Dermatology, Yenepoya Medical College, Deralakatte, Mangalore, India
5Department of Pathology, Yenepoya Medical College, Deralakatte, Mangalore, India
- *Corresponding Author:
- Nivedita L. Rao
Department of Biochemistry
Yenepoya Medical College
Deralakatte, Mangalore 575 018
Karnataka, India
Accepted date: September 11 2011
Abstract
C –reactive protein (CRP), an acute-phase reactant and a non-specific indicator of inflam-mation, has been identified as a saliva-based inflammatory biomarker. The objective of the study was to estimate salivary CRP levels in patients with acute urticaria. The study in-cluded 25 patients diagnosed with acute urticaria and 20 healthy age, sex- matched controls. CRP levels in saliva were estimated using an Enzyme- Linked ImmunoSorbent Assay me-thod with enhanced sensitivity. Saliva CRP levels were significantly elevated in patients with acute urticaria compared to healthy controls (p < 0.0005). The rise in salivary CRP level observed in acute urticaria conceivably reflects the presence of an underlying acute phase inflammatory process. Saliva CRP levels appear to have the potential to serve as non-invasive markers of systemic inflammatory status in patients with acute urticaria.
Keywords
C-reactive protein, estimation, saliva, acute urticaria, inflammatory marker
Introduction
Acute urticaria is a common skin disorder, characterized by erythematous, circumscribed, and pruritic wheals, of less than 6 weeks duration. The skin lesions are the result of mediator release from cutaneous mast cells [1,2]. C-reactive protein (CRP), the prototypical acute-phase reac-tant to infections and inflammation in human beings, has been used as a non-specific clinical indicator of acute in-fections and response to treatment, and to assess systemic inflammatory status in chronic diseases [3,4]. Previous studies have revealed elevated serum CRP levels in pa-tients with acute urticaria [5,6] but, data on saliva CRP levels in acute urticaria, are lacking. Saliva has been shown to contain systemically-derived biomarkers of in-fectious diseases [7,8]. Saliva assay systems however, require high sensitivities for the detection of biomarkers present at low, but still patho-physiologically relevant, concentrations in saliva. In the present study a newly developed Enzyme- Linked ImmunoSorbent Assay (ELISA) method with a low limit of detection for CRP was used for its estimation in saliva. Use of saliva for CRP estimations in acute urticaria, represents a non invasive and novel approach for inflammation assessment. The objective of the present study was to estimate saliva CRP levels in patients with acute urticaria.
Materials and Methods
Study group: Patients were recruited from K.S. Hegde Charitable Hospital, Deralakatte, Mangalore, Karnataka, India. The study comprised 25 patients (15 M, 10 F; age range, 18–55) diagnosed with acute urticaria. All subjects had disease duration of less than 6 weeks. Only patients on whom actual urticarial lesions were observed were included.
Control group: It consisted of 20, age and sex- matched healthy subjects.
Exclusion criteria for the study were- chronic urticaria, urticaria-vasculitis, existence of any comorbid cardiac, autoimmune, infectious, musculoskeletal or malignant disease, oral disease, alcohol abuse, pregnancy, lactation and a recent history of operation or trauma. No individual was following any drug regimen.
Ethics
The procedures followed were in accordance with the ethical standards of the Institutional Human Ethics Com-mittee and with the Helsinki Declaration. The study was approved by the Institutional Ethics Committee. Before initiation of the study, voluntary consent was obtained from each subject.
Collection of saliva samples
Unstimulated, whole saliva was collected by passive drooling as described previously [9] at least 2 hours after any food intake. Briefly, after 3 - 4 rinses of the mouth with water, saliva was allowed to accumulate in the floor of the mouth for approximately 2 min and repeatedly ex-pectorated into an ice-chilled polypropylene vial to collect about 2 ml. Following collection the samples were stored below -20º C until their analyses.
Estimation of C-reactive protein in saliva
The concentration of CRP in saliva was estimated by Enzyme- Linked ImmunoSorbent Assay (ELISA) using Sa-limetrics C- reactive protein ELISA kit (PA, USA).
Statistical analysis
Data were analyzed with SPSS-17.00 using Student’s t test (unpaired). P values lower than 0.05 were considered to be statistically significant.
Results
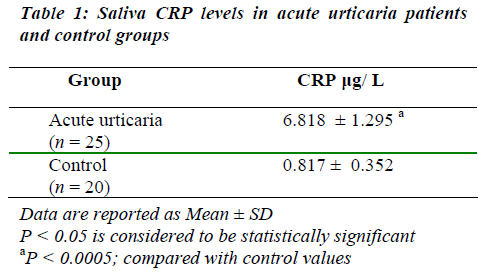
Mean value for saliva CRP concentrations in acute urti-caria patients was significantly high when compared to the control group (Table 1).
Discussion
Elevated saliva CRP levels in acute urticaria patients demonstrated in our study in the apparent absence of oral, infectious or inflammatory comorbidities, reflect underly-ing acute phase reactions in these patients. These results of CRP in saliva are in concordance with previous reports of elevated serum CRP levels in patients with acute urti-caria [6] and in recurrent urticaria [10]. According to the knowledge of the authors in this study, there are no other reports in literature, on CRP estimations in saliva of acute urticaria patients. Rise in CRP levels demonstrated in pa-tients with acute urticaria could be related to previously described elevations of serum interleukin 6 (IL6), a cyto-kine felt to be key in promoting CRP elevations [5].
Regarding the matter of association between serum and saliva CRP levels, positive correlation between porcine serum and salivary CRP, has been previously reported [11]. C-reactive protein in human saliva measured by application of a microchip assay system also showed positive correlation with serum CRP estimated by ELISA [12]. Increased serum CRP levels in patients with acute urticaria described in previous reports [5,6] and salivary CRP levels demonstrated in our study, presumably indicate a positive association between the levels.
The concentrations of most molecules present in saliva are usually one tenth to one thousandth of those in blood [7]. The estimated mean value for saliva CRP concentra-tion in acute urticaria in the present study (6.818 μg/ L; Table 1), is observed to be approximately 2000-fold lower than the previously reported mean value for serum CRP in recurrent urticaria (13.9 mg/ L) [10].
The acute phase reaction is in most circumstances a good indicator of inflammatory activity and tissue damage. CRP is a direct and quantitative measure for the acute phase reaction and due to its fast kinetics provides adequate information of the actual situation. Therefore, although CRP is a non - specific marker of an inflammatory process, it can be used as a ‘screening’ indicator for early assessment of inflammatory status of the patients and to stratify risks for further follow-up or investigation by more specific tests. Saliva-based CRP estimation in patients is advantageous as collection of saliva may be done by procedures that are considered to be non-invasive, painless and convenient.
In the present study we have demonstrated significantly increased CRP levels in saliva of acute urticaria patients compared to healthy controls. The rise in salivary CRP level observed in acute urticaria conceivably reflects the presence of an underlying acute phase inflammatory process. Saliva CRP levels appear to have the potential to serve as markers of systemic inflammatory status in patients with acute urticaria.
Acknowledgement
The authors thank Ms. Neevan D’Souza, Assistant professor, Dept. of Community Medicine, Yenepoya Medical College, Mangalore for carrying out statistical analysis of data.
References
- Kaplan AP. Urticaria and angioedema in Allergy. W.B. Saunders Co., New York 1997; pp 573–592.
- Soter NA. Acute and chronic urticaria and angioedema. J Am Acad Dermatol 1991; 25:146 –154.
- McPherson RA. Specific proteins in Clinical Diagnosis and Management by Laboratory Methods. W.B. Saun-ders Co., New York 1996; pp 237–252.
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M. Markers of inflammation and cardiovascular disease: application to clinical and public health practice. A statement for healthcare pro-fessionals from the Centers for Disease Control and Prevention and the American Heart Association. Circu-lation 2003; 107: 499 – 511.
- Fujii K, Konishi K, Kanno Y, Ohgou N. Acute urti-caria with elevated circulating interleukin-6 is resistant to anti-histamine treatment. J Dermatol 2001; 28: 248–250.
- Sakurai M, Oba M, Matsumoto K, Tokura Y, Furuka-wa F, Takigawa M. Acute infectious urticaria: clinical and laboratory analysis in nineteen patients. J Dermatol 2000; 27: 87– 93.
- Malamud D. Saliva as a Diagnostic Fluid. Br Med J 1992; 305: 207–208.
- Mandel I. D. A Contemporary View of Salivary Re-search. Crit Rev Oral Biol Med 1993; 4: 599–604.
- Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci 1993; 694: 72–77.
- Lin RY. Elevated C-Reactive Protein (CRP) Levels In Patients With Recurrent Urticaria and/or Angioedema. The Internet Journal of Asthma, Allergy and Immunol-ogy 2002; 2: Number 1.
- Gutiérrez AM, Martínez-Subiela S, Eckersall PD, Cerón JJ. C-reactive protein quantification in porcine saliva: A minimally invasive test for pig health moni-toring. The Veterinary Journal 2009; 18: 261–265.
- Christodoulides N, Mohanty S, Miller CS, Langub MC, Floriano PN, Dharshan P, Ali MF, Bernard B, Romanovicz D, Anslyn E, Fox PC, McDevitt JT. Ap-plication of microchip assay system for the measure-ment of C-reactive protein in human saliva. Lab Chip 2005; 5: 261 –269.