Research Article - Biomedical Research (2017) Volume 28, Issue 2
Safranal inhibits the migration and invasion of human oral squamous cell carcinoma cells by overcoming epithelial-mesenchymal transition
Shu-Ping Zhang1#, Jiu-Ning Huang1#, Nuo Jin2, Xue-Lei Wang1, Chao-Chao Jin3*1Department of Radiation oncology, Yantai Affiliated Hospital of Binzhou Medical University, Shandong, 264100, China
2Department of General Surgery,Huangshi Central Hospital, Huangshi, 435000, Hubei, China
3Department of Oncology, Huangshi Central Hospital, Huangshi, 435000, Hubei, China
#These authors contribute equally to this work.
Accepted date: June 30, 2016
Abstract
Safranal, a bioactive component of a traditionally used spice and condiment saffron possesses a wide spectrum of biological properties, including antitumor activities. It has been shown to inhibit the proliferation and growth of prostate cancer cells by inducing apoptosis. However, the ability of safranal to modulate the Epithelial-Mesenchymal Transition (EMT) which is indispensible to the invasiveness and metastasis of oral squamous cell carcinoma remains to be elucidated. The aim of this study was to determine whether safranal affects EMT in oral squamous cell carcinoma cells. The results showed that safranal not only inhibited oral squamous cell carcinoma cell proliferation, migration and invasion in a dose-dependent manner but also modulated the expression of EMT-related proteins E-cadherin and vimentin which are indespensible to cellular motility, invasiveness and metastasis. Taken together, our results indicate that safranal suppresses oral squamous cell carcinoma cell migration and invasion by modulating the expression of EMT proteins. The study thus suggests that safranal may serve as a potential anticancer agent for oral squamous cell carcinoma.
Keywords
Oral squamous cell carcinoma, Safranal, Epithelial-mesenchymal Transition, Migration and invasion.
Introduction
Epithelial-mesenchymal transition (EMT) is a fundamental developmental program associated with the progression of various cancer types [1,2]. During cancer pathogenesis, this process leads to the loss of epithelial characters by cells, which acquire a mesenchymal phenotype. At molecular level, EMT is associated with the downregulation of epithelial markers like E-cadherin and up-regulation of mesenchymal markers like vimentin and N-cadherin [3]. Thus cells lose their defined cellcell and cell-extracellular matrix contacts which potentiates their migration and invasion [4]. Therefore, EMT may serve as a promising therapeutic target to overcome the invasion and metastasis of cancer cells.
Natural products as chemopreventive agents may serve as alternative and safe cancer treatments [5] when compared to current anti-cancer therapies, which only offer modest benefits with a wide variety of side effects [6]. Saffron (Crocus sativus, L.), a traditionally used spice and condiment has been shown to possess potential health benefits. Safranal is a characteristic constituent of saffron which exhibits various biological properties including in vitro and in vivo anticarcinogenic and antitumor activities [7,8]. Recently safranal has been shown to inhibit the proliferation and growth of prostate cancer cells by inducing apoptosis [9]. However, little is known about the action of safranal on the migration and invasion of oral squamous cell carcinoma cell cancer cells which needs to be explored. In the present study, we demonstrated that the migration and invasion of oral squamous cell carcinoma cells could be inhibited by safranal. It was observed that safranal mediates such effects by overcoming Epithelial-mesenchymal transition of oral squamous cell carcinoma cells.
Materials and Methods
Materials
DMEM and FBS were procured from Gibco, USA. Penicillin was obtained from Invitrogen, USA. MTT, DMSO and Safranal were purchased from Sigma Aldrich (St. Louis, MO, USA). Epithelial-Mesenchymal Transition antibody sampler kit was procured from Cell Signalling and Technology (Beverly, MA, USA) whereas as anti-rabbit goat secondary antibody was purchased from Sigma Aldrich (St. Louis, MO, USA).
Cell culture
HSC-3 oral squamous cell carcinoma cells were cultured in DMEM (Gibco), supplemented with 10% fetal bovine serum (Gibco), 250 IU/ml penicillin (Invitrogen) under standard culture conditions at 37°C in 5% CO2 humidified incubator. The medium was changed regularly and the cells were subcultured every 3-4 days.
Cell proliferation assay
MTT assay is a standard test to measure the cell viability and is based on the conversion of MTT to formazan crystals by active mitochondrial dehydrogenases. Briefly, cell suspension containing about 2 × 104 cells was seeded into each well of a 96 well plate and allowed to attach for 24 h. Safranal, prepared in DMEM was added to the wells at a concentration of (10, 20, 40, and 60, 80 and 100 μM) for 48 h. After treatment, 100 μl of MTT (Sigma Aldrich) solution was added to each well at a concentration of 0.1 mg/ml (dissolved in PBS). After 4 h of incubation at 37°C in dark, the solution was removed and 100 μl of DMSO was added to solubilize the produced formazan. Absorbance was measured at 570 nm using an automated microplate reader (Bio-Rad 550). The results were depicted as the percentages relative to the controls. The percentage proliferation inhibition rate was calculated as = (1–OD sample/OD control) × 100%.
Western blotting
Cells were lysed using ice cold RIPA buffer consisting of 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% Na-deoxycholate and supplemented with protease inhibitor cocktail (Roche). The protein concentration was determined by BCA method (Thermo pierce) and thirty micrograms was loaded on a polyacrylamide gel (10%-12%) followed by transfer to PVDF membrane. The membrane was then blocked with 5% non-fat dried milk in PBS at room temperature for 2 h, incubated with primary antibodies against E-cadherin, vimentin and GAPDH at 4°C overnight. Finally, the blots were hybridized with secondary goat anti-rabbit antibodies for 1 h at room temperature and developed using an enhanced chemiluminescence detection system (Amersham).
Wound healing assay
HSC-3 cells were platted in 24-well plates and grown to confluence. The media was then removed and a scratch was made using 100 μl pipette tip. This was followed by washing with PBS and treatment with sub-toxic concentrations of safanal for 24 h. Wounds were observed and photographed before (0 h) and after treatment (48 h) using a phase contrast microscope fitted with digital camera. Wound healing was calculated as the percentage of the initial wound until total wound closure at 48 time point. The assay was repeated three independent times.
Transwell invasion assay
104 HSC-3 cells in 0.5 ml of serum-free medium containing safranal at the indicated concentration were seeded in matrigel coated transwell chambers which were placed in 24 well plates containing 10% FBS-supplemented DMEM. After 48 h, unmigrated cells that remained in the upper chamber were removed by wiping the top of the insert with a cotton swab and the cells on the underside of the membrane were fixed with 100% methanol and stained with crystal voilet. The membranes were mounted on glass slides, and numbers of cells in three randomly chosen high-power fields were counted. The assay was repeated three independent times.
Results
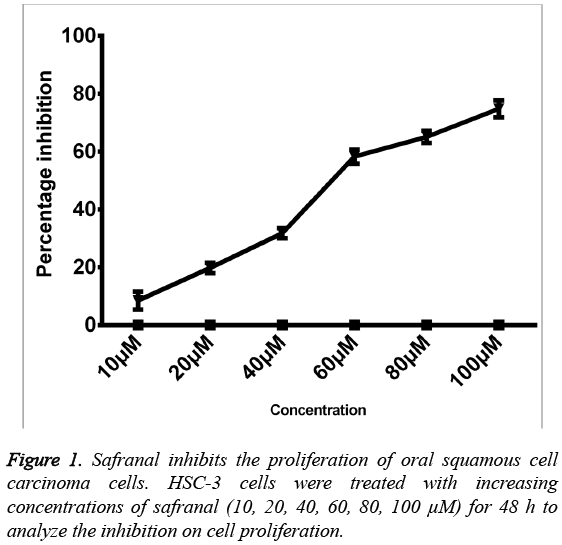
Safranal inhibits the proliferation of HSC-3 oral squamous cell carcinoma cells
The effect of safranal on the proliferation was investigated on human oral squamous cell carcinoma cells. HSC-3 cells were cultured in presence of different concentrations of safranal (10100 μM) for 48 h, and the viability was measured by MTT assay. The results demonstrated that all the tested concentrations of safranal exhibited a significant growth inhibition. The proliferative abilities of HSC-3 cells decreased in presence of safranal in a dosedependent manner with IC50 value of 56.24 μM after 48 h of incubation. Furthermore, the results showed that treatment with safranal at concentrations such as 10, 20 and 40 μM exhibited least cytotoxic effects on HSC-3 cells (Figure 1). Therefore, lower concentrations of safranal, without cytotoxic effects on HSC-3 cells, were used in subsequent experiments.
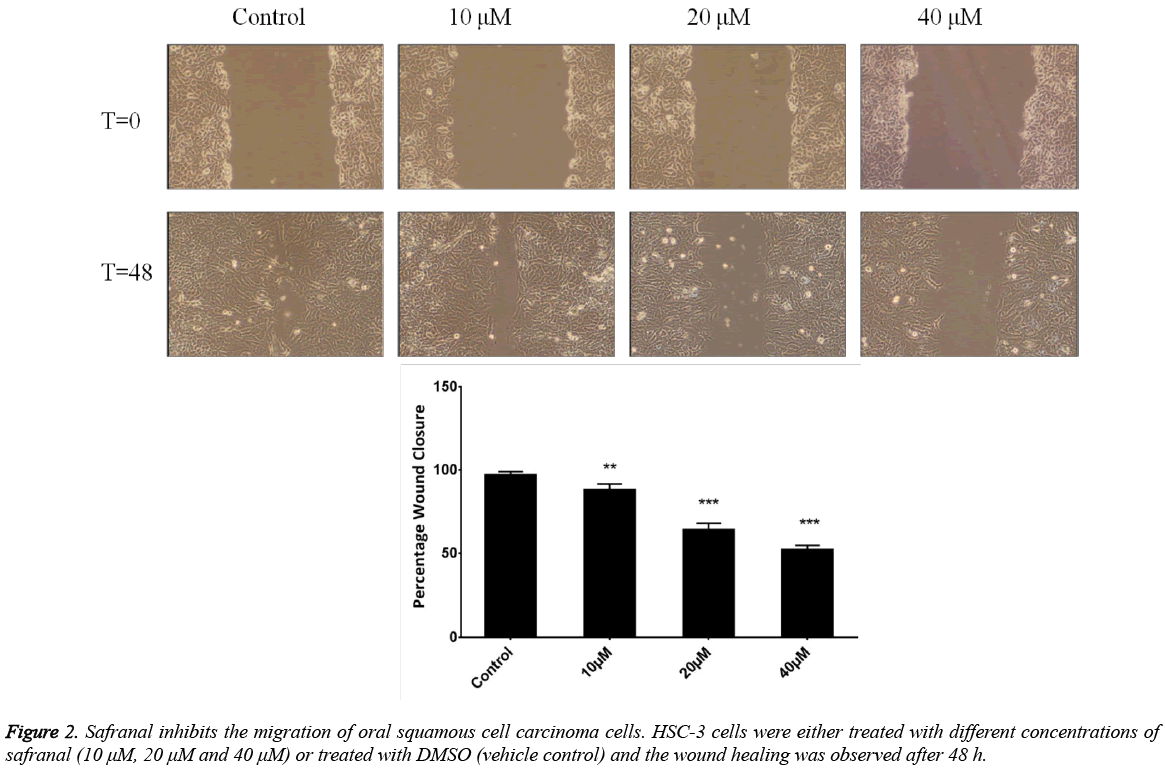
Safranal suppresses the migration of HSC-3 oral squamous cell carcinoma cells
To determine whether safranal can inhibit human prostate cancer metastasis, we first evaluated its effect on the migration of HSC-3 cells. A wound-healing assay was performed in which HSC-3 cells were grown to confluency and a line was made by scratching the cell monolayer with a pipette tip. This was followed by the growth of cells in sub-toxic concentrations of safranal for 48 h. Results were presented as the distance covered by the cells in the wound relative to initial time point when the scratch was made and expressed as percentage wound closure. It was observed that safranal significantly overcame migratory abilities of HSC-3 cells in comparison to control, with 40 μM and 20 μM concentrations, being slightly potent than 10 μM concentration (Figure 2). Taken together, these results suggest that safranal significantly inhibited migration of HSC-3 cells which was reflected as reduced closure of the wound with safranal treatment.
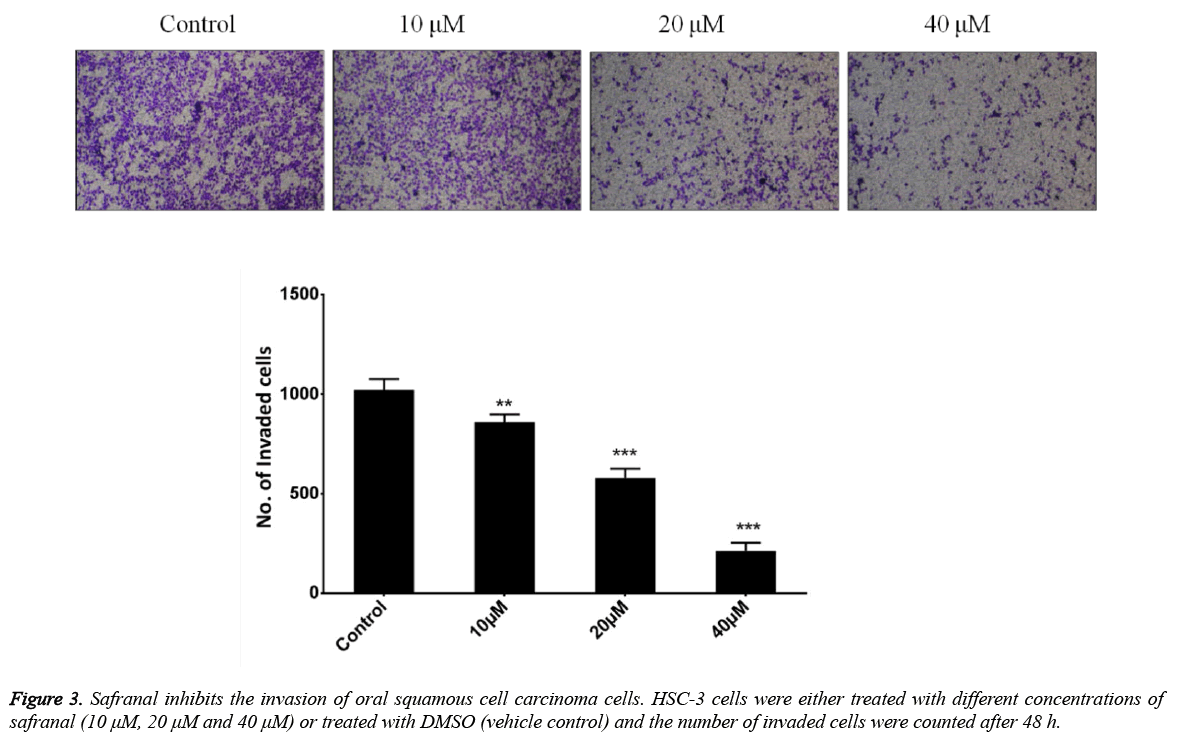
Safranal inhibits the invasiveness of HSC-3 oral squamous cell carcinoma cells
Invasiveness is the inherent capability of the tumor cells and constitutes an important process necessary for cancer metastasis. To evaluate the effect of safranal on the invasion of oral squamous cell carcinoma cells, HSC-3 cells were treated with safranal at the indicated concentration in transwell chambers coated with matrigel for 48 h. As shown in (Figure 3), it was observed that the treatment of HSC-3 cells with safranal significantly reduced their invasiveness. The effect was found to be dose dependent with 40 μM and 20 μM concentrations showing better inhibition than 10 μM concentration of safranal.
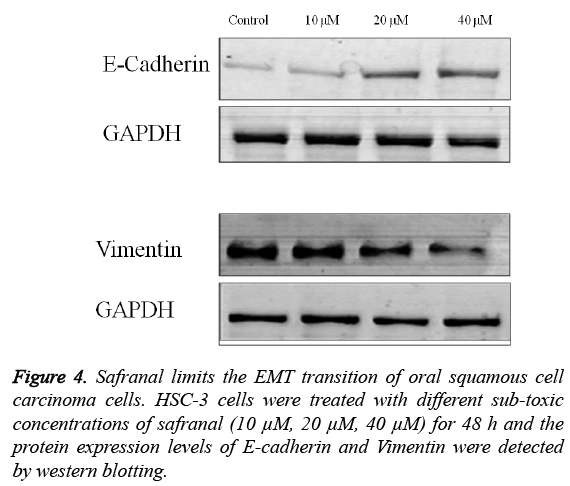
Safranal limits the Epithelial-mesenchymal transition of HSC-3 oral squamous cell carcinoma cells
The role of safranal in regulating the migration and invasion of HSC-3 cells was further evidenced by evaluating the expression of mediators of EMT including E-cadherin, vimentin and Snail. As shown in Figure 4, treatment of HSC-3 cells with different concentrations of safranal for 48 h had a considerable effect on the expression of the EMT markers.
It was observed that there was an increase in the expression of E-cadherin (epithelial marker) with corresponing decrease in expression of vimentin (a mesenchymal marker). Taken together these results indicate that the safranal inhibits the metastatic potential of HSC-3 cells by overcoming their EMT.
Discussion
Compounds isolated from natural sources or their ingredients constitute over 50% of the drugs reported for anticancer activity in clinical trials [10,11]. Dietary factors such as spices represent an attractive source of these agents, which have shown remarkable anti-carcinogenic properties both on tissue culture and on tumor cells in vivo [12,13]. Safranal, an active constituent of Saffron has been shown to exhibit inhibitory effects on the proliferation and growth of various cancer cells [7,8]. However studies evaluating the effect of safranal on the metastasis of cancer cells particularly oral squamous cell carcinoma have rarely been done. Therefore the present study was carried out to evaluate the potential of safranal in overcoming the migration and invasiveness of human oral squamous cell carcinoma.
In present study we observed that safranal was highly potent in inhibiting the proliferation of HSC-3 cells. This effect on the growth of cells was found to be dose dependent with IC50 value of 56.24 μM. The essence of safranal as an agent that could disrupt the metastatic process in the oral squamous cell carcinoma progression was tested by migration and invasion assays using HSC-3 cells. It was observed that safranal could efficiently overcome the migration and invasiveness of HSC-3 cells when used at sub-toxic concentrations. These data was further substantiated by evaluating the expression of EMT markers, E-cadherin and vimentin under treatment with safranal by western blotting. We found that safranal increased the expression of the epithelial marker, E-cadherin in HSC-3 cells in a dose dependent manner with corresponding decrease in the expression of a mesenchymal marker, vimentin by safranal treatment. The finding points at the role of safranal in impeding the EMT in HSC-3 cells and thus provides an evidence for the potential use of safranal against oral squamous cell carcinoma progression.
References
- Franco-Chuaire ML, Magda Carolina CS and Chuaire-Noack L: Epithelial-mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin 2013; 54: 186 205.
- De Craene B, Berx G: Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013; 13: 97-110.
- Borthwick LA, Parker SM, Brougham KA. Epithelial to Mesenchymal Transition (EMT) and airway remodelling after human lung transplantation. Thorax 2009; 64: 770-777.
- Evdokimova V, Tognon CE, Sorensen PH: On translational regulation and EMT. Semin Cancer Biol 2012; 222: 437-445.
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003; 3: 768-780.
- Fryer RA, Galustian C, Dalgleish AG and Dalgelish AG. Recent advances and developments in treatment strategies against pancreatic cancer. CurrClinPharmacol 2009; 4: 102-112.
- Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). ExpBiol Med 2002; 247: 20-25.
- Samarghandian S, TavakkolAfshari J, Davoodi S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. ApplBiochemBiotechnol 2011; 164: 238-247.
- SaeedSamarghandian, Mahmoud M. Shabestari DNA fragmentation and apoptosis induced by safranal in human prostate cancer cell line Indian Journal of Urology 2013; 29 :177-183
- Cragg GM, Newman DJ. Antineoplastic agents from natural sources: Achievements and future directions. Expert OpinInvestig Drugs 2000; 9: 2783-97.
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005; 100: 72-79.
- Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin 2004; 54: 150-180.
- Winterhalter P, Staudinger M. Saffron- renewed interest in an ancient spice. Food Rev Int 2000; 16:39-59.