Research Article - Biomedical Research (2020) Volume 31, Issue 6
Safety and efficacy of the honghuaruyi pill for relieving chronic pelvic pain: A multiple center and randomized controlled study.
Yanxia Liu1, Xin Xu2, Jimaocairang3, Zhongming Zhou4, Bei Lv5, Min Xia6, Baoli Cao7, Xinpei Zhang8,Shuyan Ma9, Zhe Jin1*
1 Beijing University of Chinese Medicine and Pharmacology Dongfang Hospital, Building 6, Fangxingyuan District 1, Fangzhuang, Fengtai District, Beijing, 100078, China
2 Beijing Traditional Chinese Medicine Hospital of Beijing Capital Medical University, No. 23, Back Street of Art Museum, Dongcheng District, Beijing, 100010, China
3 Tibetan Medicine Hospital of Qinghai Province, No. 97, East Road of South Mountain, Jianxin Lane, Chengdong District, Xining, Qinghai Province, 810007, China
4 Traditional Chinese Medicine Hospital of Hubei Province, No. 34, Yanzhi Road, Wuchang District, Wuhan City, Hubei, 430074, China
5 Wuxi People's Hospital, No.299, Qingyang Road, Wuxi, Jiangsu Province, 214023, China
6 Chongqin Traditional Chinese Medicine Hospital, No. 12, Zhongshan Fourth Road, Yuzhong District, Chongqing, 400015, China
7 Tianjin Nankai Hospital, No. 102, Sanwei Road, Nankai District, Tianjin, 300199, China
8 Nanyang Nanshi Hospital, No. 1081, Zhongzhou West Road, Wolong District, Nanyang, Henan Province, 473065, China
9 Daqing People's Hospital, No.213, Jianshe Road, Longfeng District, Daqing City, Heilongjiang Province, 163316, China
- *Corresponding Author:
- Zhe Jin
Beijing University of Chinese Medicine and Pharmacology Dongfang Hospital
Fengtai District
Beijing
China
Abstract
Honghuaruyi (HHRY) pill is considered as an effective treatment for Chronic pelvic pain (CPP) with the efficacy of promoting qi and blood circulation but lack of high-quality clinical evidence. This study is to evaluate the efficacy and safeties of HHRY pill.280 patients were randomly divided into two groups to receive HHRY pill or placebo. Pain relief rate, curative effect of disease as well as qi stagnation and blood stasis symptom in TCM, clinical signs of CPP, adnexal masses size and amount of fluid and EQ-5D instruments were evaluated. After treatment, pain relief rate was significantly higher (P<0.05). There was also significant difference (P<0.0001) of curative effect in both disease and symptom. The score of low abdominal pain, etc. were significantly lower (P<0.05). The score of tenderness caused by limited uterine activity and thickening or with adnexal masses of attachments were significantly different (P<0.05). The increased size of adnexal mass in HHRY pill group was 0.53 ± 8.19 and was 0.80 ± 4.28 in placebo group. The decreasing amount of fluid in HHRY pill group was 0.53 ± 2.63 and was 1.10 ± 3.40 in placebo group. Pain, anxiety and EQ-VAS score were significantly improved (P<0.05). No serious adverse reactions occurred. This study indicated HHRY pill would reduce patients’ chronic pelvic pain, improve corresponding clinical signs, symptoms in TCM and enhance the quality of life. However, more biochemical indicators are needed to assess the effect.
Keywords
Chronic Pelvic Pain, Qi stagnation and blood stasis, Traditional Chinese medicine, Honghuaruyi pill.
Abbreviations
CPP: Chronic Pelvic Pain; PID: Pelvic Inflammatory Disease; TCM: Traditional Chinese Medicine; HHRY pill: Honghuaruyi pill; GMP: Good Manufacturing Practices; VAS: Visual Analogue Scale; FAS: Full-Analysis Set; PPS: Per-Protocol Population Set; SS: Safety Set; IL-2: Inflammatory Cytokines Interleukin-2; TNF-α: Tumor Necrosis Factor; ICAM-1: Intercellular Adhesion Molecular.
Introduction
Chronic Pelvic Pain (CPP) is pain in the pelvic area that lasts for 6 months or longer. Chronic pain can come and go, or it can be constant. Sometimes chronic pelvic pain follows a regular cycle. For example, it may occur during menstruation. Constant pain will bring not only physical but also psychological burden to patient. CPP can be caused by a variety of gynecological diseases and sequelae Pelvic Inflammatory Diseases (PID) is the most common one [1]. One study estimated that for females with PID between 20 to 24 years of age, 18% would eventually develop chronic pain [2]. At present, the treatment of CCP mainly includes pain-relieving drugs therapy, physical therapy (acupuncture, acupressure, and nerve stimulation), nutrition therapy (vitamin B1 and magnesium) and surgical treatment (cutting or destroying nerves blocks pain signals from reaching tissues and organs) [1,3].
Traditional Chinese Medicine (TCM) has advantage in relieving pain, especially chronic pain. According to the theory of traditional Chinese Medicine, if the veins with blood stasis people would feel pain, especially the stabbing pain. Besides acupuncture mentioned above, herbs with the function of blood circulation promoting and blood stasis dissipating are also good at pain reducing. The formula Honghuaruyi (HHRY) pill has been patented (Chinese medicine approval number: Z20027000) and consists of 26 drugs which might remove blood stasis. They are Flos Carthami (Honghua), Stigma Croci (Xihonghua), Rhizoma Podophyllum Hexandrum (Taoerqi), Fructus Chebulae (Hezi, Radix et Rhizoma Rubiae (Zangqiancao), Cortex Cinnamon (Rougui), Herba Corydalis Impatiens(Baxiaga), Racemose Jurinea (Zangmuxiang), Fructus Coriandri Sativi (Yansuiguo), Lignum Dalbergiae Odoriferae (Jiangxiang), Powder of Bear Gall (Xiongdanfen), Radix Onosmae (Zangzicao), Bright Salt (Guangmingyan), Radix Himalayan Purple Jasmine (Ximalayazimoli), Bangga, Fructus Piperis(Hujiao), Zaocys(Huasherou), Herba Corydalis(Aizijin), Fructus Phyllanthi (Yuganzi), Cream of Fructus Hippophae (Shajigao), Sal Ammoniac (Naosha), Lacca (Zicaorong), Fructus Lycii (Gouqizi), Lignum Aquilariae Resinatum (Chenxiang), Potassium Nitrate (Huoxiao). Modern researches indicated that HHRY pill can alleviate the inflammation and adhesion of pelvic by regulating the expression of inflammatory cytokines in serum and intercellular adhesion molecules in endometrium [4]. A small sample size, multi-center, randomized, double-blind clinical trial also demonstrated the combination of HHRY pill and antibiotics can effectively relieve abdominal pain caused by pelvic inflammatory disease. Its mechanism may be related to the reduction of TNF-α and the up-regulation of IL-2 expression [5,6]. However, there is still lack of a clinical trial with bigger sample size and longer period. Therefore, we designed a multi-center, prospective, randomized, double-blind, and placebo-controlled clinical trial to evaluate the safety and efficacy of the HHRY pill.
Methods
Setting and study population
This study was a multiple center, prospective, randomized, double-blind and placebo controlled clinical pilot trial with two parallel groups. The study received ethical approval from the Ethics Committee of Beijing University of Chinese Medicine and Pharmacology Dongfang Hospital (Ethical Review no. Y2016-001) and was registered with Clinical Trials.gov (ClinicalTrials. gov ID: ChiCTR-IPR-15006945). Other clinical centers were Tibetan Medicine Hospital of Qinghai Province, Beijing Traditional Chinese Medicine Hospital of Beijing Capital Medical University, Traditional Chinese Medicine Hospital of Hubei Province, Wuxi People's Hospital, Chongqin Traditional Chinese Medicine Hospital, Tianjin Nankai Hospital, Nanyang Nanshi Hospital and Daqing People's Hospital.
All participants were recruited by posters in the hospital or in the hospital’s WeChat official account advertises. All patients in the screening session were informed of the protocol and signed a consent form. A total of 280 eligible patients who had chronic pelvic pain caused by sequelae pelvic inflammatory diseases and were diagnosed as qi stagnation and blood stasis in TCM Table 1 shows the detailed eligibility and exclusion criteria) were randomly divided into the HHRY pill group and the placebo group according to a 3:1 ratio and received a three menstrual cycle treatment.
| Inclusion criteria | |
|---|---|
| 1 | Meet the diagnostic criteria for sequelae of pelvic inflammatory disease and chronic pelvic pain lasts for more than 6 months |
| 2 | Meet the diagnostic criteria for qi stagnation and blood stasis in TCM |
| 3 | Women who are between the ages of 20 and 50 and married or have sexual life |
| 4 | Menstrual cycle is 28 to 35 days |
| 5 | Chronic pelvic pain level: (VAS) ≥ 40mm |
| 6 | Subjects volunteer to sign the informed consent and participate the experiment and the process of obtaining informed consent meets the Good Clinical Practice (GCP) regulations. |
| Exclusion Criteria | |
| 1 | Pregnancy,Preparing pregnancy within half a year or Lactating woman |
| 2 | With symptoms caused by gynecological tumors, trichomonas vaginitis, vulvovaginal candidiasis, bacterial vaginosis, acute cervicitis, pelvic inflammatory disease, endometriosis, adenomyosis, pelvic venous stasis syndrome, Interstitial cystitis (IC) and so on. |
| 3 | Serum CA-125 ≥ 35U/ml, or Erythrocyte sedimentation rate (ESR)>25mm/h |
| 4 | Patients who have serious primary diseases of cardiovascular, liver, kidney or blood system or have mental disorders |
| 5 | Patients who have taken drugs with similar effect or undergone other relevant therapy within 2 weeks |
| 6 | Patients who are participating in other clinical trials or who have been treated with antibiotics within the recent month |
| 7 | Known history of allergy to the trial drug |
| 8 | Persons with disabilities prescribed by law (blind, deaf, dumb, mental retardation, mental disorder, physical disability) |
| 9 | Patients who are recognized by the researchers that they are inappropriate to participate the clinical trial |
| 10 | Suspected or indeed have a history of alcohol and drug abuse |
Table 1: Study inclusion and exclusion criteria.
Randomization and blinding
280 patients were randomly divided into HHRY group and placebo group at a ratio of 3:1 by random number table which was generated by SAS software version 9.2. The stochastic allocation procedure was saved in the clinical trial data management and statistical unit in Beijing University of Chinese Medicine and Pharmacology Dongfang Hospital. Stratified block randomization was concealed using a sequentially numbered and opaque envelope. Eligible patients were randomized into the HHRY pill groups or the placebo groups by obtaining medicines associated with the given medicine codes in accordance with the order of visits. Participants, investigators, statisticians, and all study staff were blinded. Only data administrators were permitted access to the unblinded data.
Interventions
All eligible patients received the basic treatment according to standard for diagnosis and treatment of pelvic inflammatory diseases (revised edited by Infectious Diseases Cooperative Group of Obstetrics and Gynecology Branch in Chinese Medical Association). For the HHRY pill group, the HHRY pill is composed of Flos Carthami (Honghua), Stigma Croci (Xihonghua), Rhizoma Podophyllum Hexandrum (Taoerqi), Fructus Chebulae (Hezi , Radix et Rhizoma Rubiae (Zangqiancao), Cortex Cinnamon (Rougui), Herba Corydalis Impatiens(Baxiaga), Racemose Jurinea (Zangmuxiang), Fructus Coriandri Sativi (Yansuiguo), Lignum Dalbergiae Odoriferae (Jiangxiang), Powder of Bear Gall (Xiongdanfen), Radix Onosmae (Zangzicao), Bright Salt (Guangmingyan), Radix Himalayan Purple Jasmine (Ximalayazimoli), Bangga, Fructus Piperis(Hujiao), Zaocys(Huasherou), Herba Corydalis(Aizijin), Fructus Phyllanthi (Yuganzi), Cream of Fructus Hippophae (Shajigao), Sal Ammoniac (Naosha), Lacca (Zicaorong), Fructus Lycii (Gouqizi), Lignum Aquilariae Resinatum (Chenxiang), Potassium Nitrate (Huoxiao). For the placebo group, the color, smell and form of pill were consistent with the HHRY pill. Both HHRY pill and placebo were prepared by Gannanfoge Tibetan Medicine Company Limited according to Good Manufacturing Practices (GMP). Quality control was strictly enforced throughout the trial.
Patients were instructed to take five pills each time, twice a day on the seventh day of each menstrual cycle for twenty-one days and stop to take when menstruating. The medication period was three menstrual cycles. During the period, the investigators made four visits and investigations with the patients: six days before treatment, the end of first menstrual cycles, the end of second menstrual cycles and seven days after the end of medication. Besides, the investigators were also responsible for recording the adverse events that occurred during the medication.
Measurement
The primary outcome measure was remission rate of pain during menstrual cycle. Visual Analogue Scale (VAS) score was used to evaluate lower abdominal pain in last menstrual cycle. The secondary outcomes were as follow: (1) curative effect of disease; (2) curative effect of qi stagnation and blood stasis; (3) the score of syndromes in TCM; (4) score of common clinical signs in CPP; (5) adnexal masses size and amount of fluid; (6) score of EQ- 5D Instruments.
Sample size
Based on the relative researches of HHRY pill on CPP, we supposed that the pain control rate of placebo group was around 30% and the HHRY pill group was about 60%. As calculated by the PASS 11.0 software, a total sample size of 227 achieved 80% power of test and ruled out a two-sided type I error of 5% to detect a superiority margin difference of 10% in this trial. Considering a 20% loss to follow-up, the sample size was adjusted to 270. Moreover, the proportion between HHRY pill group and placebo group was set to 3:1. Thus, there were 210 in the HHRY pill group while 70 in the placebo group.
Statistical analysis
All the statistical analyses were performed with the Statistical Analysis System (SAS 9.1.3.) The statistical analysis set included Full-Analysis Set (FAS), Per- Protocol Population Set (PPS) and Safety Set (SS) (Table 2). According to the principle of intention-to- treat analysis, FAS was a randomized group with at least one intervention. For cases of earlier withdrawal, the main efficacy indicators of the corresponding evaluation points will be filled by the last observation of the forward observation (LOCF). PPS was in accordance with the inclusion criteria specified in the trial protocol and the compliance was greater than 80%. Subjects in SS were all randomized and underwent the HHRY pill at least once as well as accepting at least one safety assessment.
| HHRY pill group | Placebo group | ||||
|---|---|---|---|---|---|
| FAS | PPS | SS | FAS | PPS | SS |
| 210 | 159 | 210 | 69 | 54 | 69 |
Table 2: Size of each statistical set.
All statistical tests were performed by a two-sided test. The test level was defined as α=0.05 and the difference were statistically significant at P ≤ 0.05. There were four kinds statistical analyses in the trial. (1) The baseline information description and balance analysis: Baseline data were analyzed with standard descriptive statistics. The quantitative descriptive variables such as body weight which met the normal distribution were analyzed by the group t test. The Wilcoxon rank sum test was applied to analyze the quantitative variables which did not meet the normal distribution. Pearson chi-square test, Fisher's exact probability or rank sum test were used to analyze the qualitative variables between two groups. (2) Analysis of efficacy indicators: CMH chi-square was used to calculate the difference of pain relief rate, indicators of qi stagnation and blood stasis syndrome, etc. in two groups and 95% was set as the confidence interval. (3) Analysis of shedding: The chi-square test/Fisher exact probability method was used to compare the shedding rate.
Results
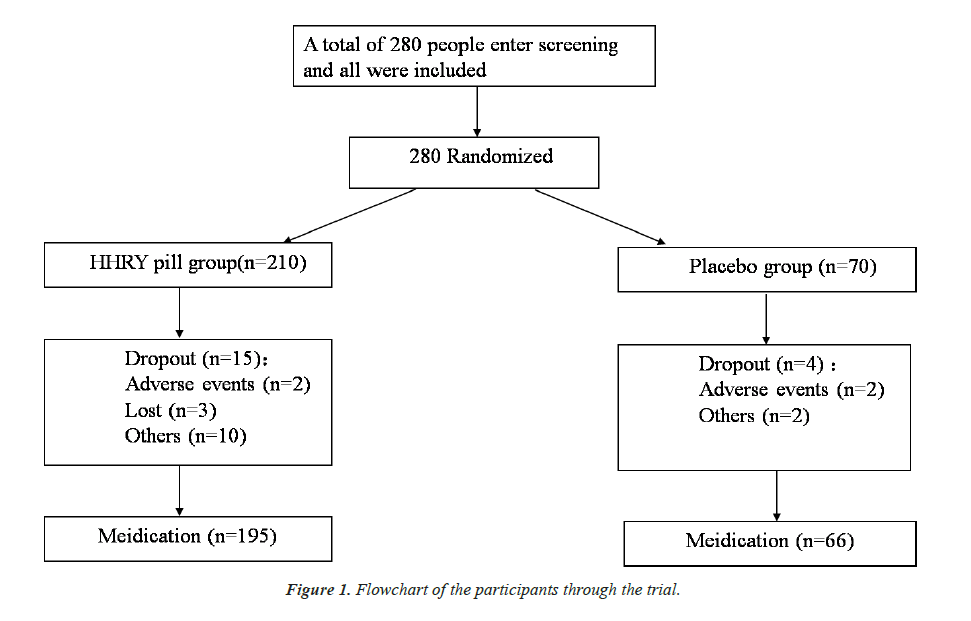
A total of 280 patients participated the study and finally 261 subjects finished the study (195 in the HHRY pill group and 66 in the placebo group) (Figure 1) There were no significant differences (P>0.05) in age, blood pressure, body temperature, respiration, heart rate, history of medication, etc. between two groups. These two groups were baseline balanced and comparable (Table 3).
| HHRY pill group (n=210) | Placebo group (n=69) | P-Value | |
|---|---|---|---|
| Basic Features | |||
| Married | 0.7529 | ||
| Yes | 200 (95.24%) | 65 (94.20%) | |
| No | 10 (4.76%) | 4 (5.80%) | |
| Age (Yr) x͞ ±s | 37.42 ±7.16 | 37.17 ±6.71 | 0.7986 |
| Height (cm) x͞ ±s | 160.98 ±4.00 | 160.68 ±4.22 | 0.5901 |
| Weight (kg) x͞ ±s | 58.74 ± 7.85 | 58.06±8.18 | 0.5376 |
| Systolic blood pressure (mmHg) x͞ ±s | 108.25 ± 8.29 | 107.72 ± 8.95 | 0.6562 |
| Diastolic blood pressure(mmHg) x͞ ±s | 73.17 ±6.53 | 73.10 ±6.70 | 0.9431 |
| Respiratory rate (beats/min) x͞ ±s | 17.89± 1.31 | 18.03 ±1.37 | 0.4379 |
| Body temperature (°C) x͞ ±s | 36.42 ± 0.29) | 36.46±0.24 | 0.3483 |
| Heart rate (beats/min) x͞ ±s | 71.73 ±8.90 | 71.13 ±7.41 | 0.6122 |
| Past history | |||
| Sequel of chronic pelvic inflammation | 1.0000 | ||
| Yes | 210 (100.00%) | 69 (100.00%) | |
| No | 0 (0.00%) | 0 (0.00%) | |
| Past history of allergy | 0.6002 | ||
| Yes | 3 (1.43%) | 2 (2.90%) | |
| No | 207(98.57%) | 67 (97.10%) | |
| Past history of medication | 0.1985 | ||
| Yes | 19 (9.05%) | 10 (14.49%) | |
| No | 191(90.95%) | 59 (85.51%) |
Table 3: Baseline characteristics (FAS).
Pain relief rate after medication
The first end of menstrual cycle’ s pain relief rate in HHRY pill (The desreased VAS score ≥ 30%) was 15.71% while the placebo group was 5.80%oThere was significantly difference between two groups (P<0.05). The second end of menstrual cycle’s pain relief rate in HHRY pill (The decreased VAS score ≥ 30%) was 61.90% while the placebo group was 31.88%oThere was significantly difference between two groups (P<0.05) which indicated that at the end of the second menstrual cycle, the treatment effect of HHRY group was better than that of the control group. After the medication, the pain relief rate (The decreased VAS score ≥30%) of HHRY pill (75.71%) was significantly higher (P<0.05) than that of the placebo group (50.72%). The rate difference (95% CI) was 24.99(11.84,38.13). The lower limit of the confidence interval was greater than the preset priority value of 10%, which meant the superior effect of HHRY pill in relieving pain was established. The result was same in FAS and PPS analysis.
Curative effect of disease
After the treatment of HHRY pill, the curative rate was 19.05%, and the marked response rate was 14.29% and the response rate was 47.14%. The curative rate of placebo group was 1.45%, the marked response rate was 8.70% and the response rate was 31.88%. There was significant difference (P<0.0001) between two groups which indicated that the curative effect of disease in HHRY pill was better than that of placebo group. The result was same in FAS and PPS analysis.
Curative effect of qi stagnation and blood stasis
After the treatment of HHRY pill, the curative rate was 7.14%, and the marked response rate was 42.86% and the response rate was 32.86%. The curative rate of placebo group was 0%, the marked response rate was 17.39% and the response rate was 33.33%. There was significant difference (P<0.0001) between two groups which indicated that the curative effect qi stagnation and blood stasis in HHRY pill was better than that of placebo group. The result was same in FAS and PPS analysis.
The score of symptoms in TCM
The score of symptoms, lower abdominal pain, lumbosacral pain, purple menstrual color, increased abdominal pain during menstruation, more leucorrhea and breast pain were significantly different between the HHRY pill group and the placebo group (P<0.05). There was no significant difference (P>0.05) in the symptoms, fixed menstrual pain and less menstrual. The analyses of FAS and PPS all supported these results (Table 4).
| FAS | PPS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HHRY Pill group | Placebo group |
Z/T | P | HHRY Pill group | Placebo group |
Z/T | P | ||
| Lower abdominal pain | |||||||||
| Before treatment | 2.42 (0.82) | 2.35 (0.76) | -0.6383 | 0.5233 | 2.43 (0.82) | 2.33 (0.75) | -0.7425 | 0.4578 | |
| After treatment | 1.15 (1.01) | 1.71(0.86) | 3.9554 | <.0001 | 1.09 (1.00) | 1.74 (0.78) | 4.1003 | <.0001 | |
| Difference | 1.27 (1.16) | 0.64(0.94) | -3.9368 | <.0001 | 1.33 (1.18) | 0.59 (0.92) | -4.0406 | <.0001 | |
| Lumbosacral pain | |||||||||
| Before treatment | 2.18 (0.58) | 2.17 (0.57) | -0.0869 | 0.9307 | 2.21 (0.62) | 2.19 (0.59) | -0.2957 | 0.7674 | |
| After treatment | 1.04 (1.00) | 1.51 (0.99) | 3.1899 | 0.0014 | 1.02 (1.00) | 1.56 (0.92) | 3.3079 | 0.0009 | |
| Difference | 1.14 (1.12) | 0.67 (1.07) | -3.2414 | 0.0012 | 1.19 (1.13) | 0.63 (1.09) | -3.3861 | 0.0007 | |
| Purple menstrual color | |||||||||
| Before treatment | 0.78 (0.41) | 0.77(0.43) | -0.2209 | 0.8252 | 0.79 (0.41) | 0.76 (0.43) | -0.5092 | 0.6106 | |
| After treatment | 0.30 (0.46) | 0.52 (0.50) | 3.3328 | 0.0009 | 0.29 (0.45) | 0.56 (0.50) | 3.5188 | 0.0004 | |
| Difference | 0.48 (0.56) | 0.25 (0.47) | -3.3529 | 0.0008 | 0.50 (0.57) | 0.20 (0.45) | -3.7166 | 0.0002 | |
| Increased abdominal pain during menstruation | |||||||||
| Before treatment | 0.86 (0.35) | 0.78 (0.42) | -1.5638 | 0.1179 | 0.86 (0.35) | 0.81 (0.39) | -0.7075 | 0.4793 | |
| After treatment | 0.33 (0.47) | 0.45 (0.50) | 1.7345 | 0.0828 | 0.30 (0.46) | 0.44 (0.50) | 1.9073 | 0.0565 | |
| Difference | 0.53 (0.50) | 0.33 (0.47) | -2.8102 | 0.0050 | 0.55 (0.50) | 0.37 (0.49) | -2.3182 | 0.0204 | |
| More leucorrhea | |||||||||
| Before treatment | 0.77 (0.42) | 0.80 (0.41) | 0.5224 | 0.6014 | 0.78 (0.42) | 0.81 (0.39) | 0.5404 | 0.5889 | |
| After treatment | 0.29 (0.45) | 0.58 (0.50) | 4.4093 | <.0001 | 0.27 (0.45) | 0.59 (0.50) | 4.2707 | <.0001 | |
| Difference | 0.48 (0.50) | 0.22 (0.42) | -3.8459 | 0.0001 | 0.51 (0.50) | 0.22 (0.42) | -3.6665 | 0.0002 | |
| Breast pain | |||||||||
| Before treatment | 1.23 (0.62) | 1.14 (0.69) | -0.9998 | 0.3174 | 1.22 (0.64) | 1.17 (0.72) | -0.5645 | 0.5724 | |
| After treatment | 0.27 (0.46) | 0.72 (0.57) | 6.1273 | <.0001 | 0.28 (0.45) | 0.74 (0.56) | 5.4153 | <.0001 | |
| Difference | 0.96 (0.68) | 0.42 (0.76) | -5.5286 | <.0001 | 0.94 (0.70) | 0.43 (0.77) | -4.5276 | <.0001 | |
| Fixed menstrual pain | |||||||||
| Before treatment | 0.78 (0.41) | 0.84 (0.37) | 1.0626 | 0.2879 | 0.75 (0.43) | 0.87 (0.34) | 1.7785 | 0.0753 | |
| After treatment | 0.22 (0.42) | 0.28 (0.45) | 0.8715 | 0.3835 | 0.20 (0.40) | 0.26 (0.44) | 0.8910 | 0.3729 | |
| Difference | 0.56 (0.53) | 0.57 (0.50) | 0.0000 | 1.0000 | 0.55 (0.54) | 0.61 (0.49) | 0.5880 | 0.5566 | |
| Less menstrual | |||||||||
| Before treatment | 0.17 (0.38) | 0.25 (0.43) | 1.3732 | 0.1697 | 0.17 (0.38) | 0.22 (0.42) | 0.8565 | 0.3917 | |
| After treatment | 0.09 (0.29) | 0.19 (0.39) | 2.2092 | 0.0272 | 0.11 (0.32) | 0.20 (0.41) | 1.6693 | 0.0951 | |
| Difference | 0.08 (0.41) | 0.06 (0.38) | -0.4243 | 0.6713 | 0.06 (0.42) | 0.02 (0.36) | -0.6059 | 0.5446 | |
Table 4: Statistical analysis result about the score of symptoms in TCM.
Common clinical signs of chronic pelvic pain
The symptoms, tenderness caused by limited uterine activity and thickening or with adnexal masses of attachments were significantly different between the HHRY pill group and the placebo group (P<0.05). The analyses of FAS and PPS all supported this result. For the uterosacral ligaments thickening and tenderness, PPS analysis indicated that HHRY pill group was significantly improved after medication (Table 5).
| FAS | PPS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HHRY Pill group | Placebo group | Z/T | P | HHRY Pill group | Placebo group | Z/T | P | |||
| Tenderness caused by limited uterine activity | ||||||||||
| Before treatment | 2.32 (0.79) | 2.35 (0.84) | 0.2330 | 0.8157 | 2.30 (0.75) | 2.37 (0.88) | 0.5910 | 0.5545 | ||
| After treatment | 0.84 (1.10) | 1.57 (1.08) | 4.7711 | <.0001 | 0.67 (0.97) | 1.48 (1.04) | 4.9026 | <.0001 | ||
| Difference | 1.49 (1.09) | 0.78 (0.98) | -4.5940 | <.0001 | 1.64 (1.05) | 0.89 (1.00) | -4.3809 | <.0001 | ||
| Thickening or with adnexal masses of left attachments | ||||||||||
| Before treatment | 1.00 (0.67) | 1.06 (0.64) | 0.6752 | 0.4995 | 0.98 (0.68) | 1.07 (0.64) | 0.8786 | 0.3796 | ||
| After treatment | 0.48 (0.66) | 0.75 (0.65) | 3.3112 | 0.0009 | 0.43 (0.62) | 0.76 (0.64) | 3.5765 | 0.0003 | ||
| Difference | 0.51 (0.64) | 0.30 (0.58) | -2.5504 | 0.0108 | 0.55 (0.66) | 0.31 (0.61) | -2.4296 | 0.0151 | ||
| Thickening or with adnexal masses of right attachments | ||||||||||
| Before treatment | 0.83 (0.77) | 0.81 (0.71) | -0.0434 | 0.9653 | 0.86 (0.78) | 0.78 (0.72) | -0.5541 | 0.5795 | ||
| After treatment | 0.36 (0.58) | 0.62 (0.69) | 3.0581 | 0.0022 | 0.36 (0.57) | 0.59 (0.63) | 2.6536 | 0.0080 | ||
| Difference | 0.47 (0.62) | 0.19 (0.55) | -3.0144 | 0.0026 | 0.50 (0.65) | 0.19 (0.48) | -3.1455 | 0.0017 | ||
| Uterosacral ligaments thickening and tenderness | ||||||||||
| Before treatment | 1.14 (1.22) | 1.01 (1.17) | -0.7334 | 0.4633 | 1.21 (1.21) | 1.11 (1.14) | -0.4300 | 0.6672 | ||
| After treatment | 0.50 (0.87) | 0.72 (1.03) | 1.6750 | 0.0939 | 0.49 (0.86) | 0.81 (1.07) | 2.0826 | 0.0373 | ||
| Difference | 0.65 (1.07) | 0.29 (0.79) | -2.5202 | 0.0117 | 0.72 (1.13) | 0.30 (0.72) | -2.5960 | 0.0094 | ||
Table 5: Statistical analysis result about the score of common clinical signs of CPP.
Adnexal masses size and amount of fluid
After with the treatment of HHRY pill, the increased size of adnexal mass was 0.53 ± 8.19 while the size of adnexal mass in placebo group increased to 0.80 ± 4.28. The decreasing amount of fluid in HHRY pill group was 0.53 ± 2.63 while that of the placebo group was 1.10 ± 3.40. Though there was difference in two groups on the change of adnexal masses size and amount of fluid, the difference was not statistically significant (P>0.05). The analyses of FAS and PPS all supported this result (Table 6).
| FAS | PPS | |||||||
|---|---|---|---|---|---|---|---|---|
| HHRY Pill group | Placebo group | Z/T | P | HHRY Pill group | Placebo group | Z/T | P | |
| The increased size of adnexal mass | ||||||||
| Before treatment | 0.23 (1.65) | 0.09 (0.75) | -0.4852 | 0.6275 | 0.23 (1.61) | 0.00 (0.00) | -1.1880 | 0.2348 |
| N(Nmiss) | 202 (8) | 68 (1) | 151 (8) | 53 (1) | ||||
| After treatment | 0.68 (7.87) | 0.91 (4.87) | 0.7991 | 0.4242 | 0.81 (8.67) | 0.47 (3.32) | 0.2999 | 0.7643 |
| N(Nmiss) | 176 (34) | 58 (11) | 145 (14) | 50 (4) | ||||
| Difference | -0.53 (8.19) | -0.80 (4.28) | -1.2078 | 0.2271 | -0.63 (9.06) | -0.47 (3.32) | -0.9165 | 0.3594 |
| The decreasing amount of fluid | ||||||||
| Before treatment | 1.40 (3.55) | 1.29 (3.21) | 0.1207 | 0.9039 | 1.49 (3.82) | 0.91 (2.54) | -0.2268 | 0.8206 |
| N(Nmiss) | 169 (41) | 59 (10) | 124 (35) | 44 (10) | ||||
| After treatment | 0.97 (2.85) | 0.24 (0.67) | -1.6325 | 0.1026 | 1.11 (3.06) | 0.18 (0.55) | -2.1280 | 0.0333 |
| N(Nmiss) | 155 (55) | 53 (16) | 127 (32) | 46 (8) | ||||
| Difference | 0.53 (2.63) | 1.10 (3.40) | 1.0999 | 0.2714 | 0.55 (2.73) | 0.75 (2.65) | 0.5411 | 0.5884 |
Table 6: Statistical analysis result of Adnexal masses size and amount of fluid.
EQ-5D instruments
In the HHRY pill group, the reduced value of pain was 0.46 ± 0.50 while that of placebo group was 0.30 ± 0.46. For anxiety, the reduced value was 0.45 ± 0.52 in HHRY pill group and 0.32 ± 0.47 in placebo group. For EQ-VAS score on overall quality of life, the increased value of HHRY pill group was 14.99 ± 8.86 while the placebo group was 12.90 ± 9.08. All these had significant different between two groups (P<0.05). However, there was no significant difference in the score of usual activities, mobility and self-care. Except the result of anxiety rating, other results were same in FAS and PPS analysis (Table 7).
| FAS | PPS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HHRY Pill group | Placebo group | Z/T | P | HHRY Pill group | Placebo group | Z/T | P | ||||||
| Pain | |||||||||||||
| Before treatment | 2.00 (0.12) | 2.01 (0.12) | 1.1526 | 0.2491 | 1.99 (0.14) | 2.00 (0.00) | 0.3318 | 0.7400 | |||||
| After treatment | 1.53 (0.50) | 1.71 (0.49) | 2.4665 | 0.0136 | 1.48 (0.50) | 1.69 (0.47) | 2.6282 | 0.0086 | |||||
| Difference | 0.46 (0.50) | 0.30 (0.46) | -2.293 | 0.022 | 0.52 (0.50) | 0.31 (0.47) | -2.550 | 0.011 | |||||
| Anxiety | |||||||||||||
| Before treatment | 1.53 (0.50) | 1.42 (0.50) | -1.5569 | 0.1195 | 1.49 (0.50) | 1.39 (0.49) | -1.2898 | 0.1971 | |||||
| After treatment | 1.08 (0.27) | 1.10 (0.30) | 0.6589 | 0.5100 | 1.04 (0.19) | 1.07 (0.26) | 1.0846 | 0.2781 | |||||
| Difference | 0.45 (0.52) | 0.32 (0.47) | -1.9762 | 0.0481 | 0.45 (0.51) | 0.31 (0.47) | -1.7880 | 0.0738 | |||||
| EQ-VAS | |||||||||||||
| Before treatment | 66.10 (9.10) | 65.16 (10.26) | -0.0156 | 0.9876 | 66.54 (9.21) | 65.83 (9.04) | -0.0309 | 0.9754 | |||||
| After treatment | 81.09 (9.18) | 78.06 (7.48) | -3.3032 | 0.0010 | 82.59 (7.21) | 78.74 (6.31) | -3.6527 | 0.0003 | |||||
| Difference | -14.99 (8.86) | -12.90 (9.08) | 2.1697 | 0.0300 | -16.05 (8.33) | -12.91 (7.69) | 2.4541 | 0.0141 | |||||
| Mobility | |||||||||||||
| Before treatment | 1.03 (0.18) | 1.03 (0.17) | -0.1742 | 0.8617 | 1.02 (0.14) | 1.02 (0.14) | -0.0109 | 0.9913 | |||||
| After treatment | 1.01 (0.10) | 1.00 (0.00) | -0.8062 | 0.4201 | 1.00 (0.00) | 1.00 (0.00) | 0.0000 | 1.0000 | |||||
| Difference | 0.02 (0.15) | 0.03 (0.17) | 0.2349 | 0.8143 | 0.02 (0.14) | 0.02 (0.14) | -0.0109 | 0.9913 | |||||
| Self-care | |||||||||||||
| Before treatment | 1.01 (0.12) | 1.00 (0.00) | -0.9916 | 0.3214 | 1.01 (0.11) | 1.00 (0.00) | -0.8185 | 0.4131 | |||||
| After treatment | 1.00 (0.00) | 1.00 (0.00) | 0.0000 | 1.0000 | 1.00 (0.00) | 1.00 (0.00) | 0.0000 | 1.0000 | |||||
| Difference | 0.01 (0.12) | 0.00 (0.00) | -0.9916 | 0.3214 | -0.8185 | 0.4131 | |||||||
| Usual activities | |||||||||||||
| Before treatment | 1.07 (0.26) | 1.06 (0.24) | -0.3823 | 0.7022 | 1.08 (0.26) | 1.06 (0.23) | -0.4902 | 0.6240 | |||||
| After treatment | 1.00 (0.07) | 1.00 (0.00) | -0.5649 | 0.5721 | 1.00 (0.00) | 1.00 (0.00) | 0.0000 | 1.0000 | |||||
| Difference | 0.07 (0.25) | 0.06 (0.24) | -0.2526 | 0.8006 | 0.08 (0.26) | 0.06 (0.23) | -0.4902 | 0.6240 | |||||
Table 7: Statistical analysis result of EQ-5D Instruments.
Adverse reactions and follow-up
During the treatment period, 44 adverse events were reported in HHRY pill group which were urinary tract abnormality (15), electrocardiography abnormality (5), vaginitis (3), high total bilirubin (3), etc. Placebo group had 4 adverse events that were urinary tract abnormality (1), electrocardiography abnormality (1), vaginitis (1) and high platelet value (1). All these adverse events were unrelated to the HHRY pill. 2 patients in the HHRY pill group withdrew from the trial due to adverse events. During the follow-up, 13 cases in the treatment group and 4 cases control dropped out.
Discussion
This study aimed to investigate the safety and efficacy of the HHRY pill in the treating chronic pelvic pain of qi stagnation and blood stasis. After medication of three menstrual cycles, there were evaluations significantly improved in HHRY pill group, such as pain relief, curative effect of CCP and qi stagnation and blood stasis symptoms lower abdominal pain or tingling, lumbosacral pain, purple menstrual color, increased abdominal pain during menstruation, more leucorrhea and breast, tenderness caused by limited uterine activity, thickening or with adnexal masses of attachments, tenderness of attachment, uterosacral ligaments thickening and tenderness, the reduced value of pain in EQ-5D instruments and EQ-VAS score on overall quality of life. Though adverse reactions occurred during treatment period, there was no liver or kidney dysfunction, and all adverse events were not induced by taking HHRY pill.
CPP is seen in as many as one-third of women with PID which brings tremendous pain to the patient both physically and mentally and greatly reduces quality of life [2]. Therefore, this study selected pain relief rate as main effective evaluation of HHRY pill. Final results indicated that HHRY pill could significantly relieve pain and obviously reduced anxiety of patients, which improved the quality of life. These results were evaluated by the EQ- 5D instruments. Besides pain, the following symptoms are usually accompanying with CCP, for example, tenderness caused by limited uterine activity, thickening or with increased adnexal masses of attachments, tenderness of attachment, uterosacral ligaments thickening and tenderness and larger amount of fluid in pelvic. Except adnexal masses and amount of amount of fluid in pelvic, HHRY pill also relieved the symptoms.
In TCM, the main pathogenesis of CCP is qi stagnation and blood stasis. Blood stasis in pelvic tend to cause the following symptoms, lower abdominal and lumbosacral pain which intensify during the menstrual cycle, change in the menstrual color, etc. The HHRY pill also proved to improve such TCM symptoms. And this was attributed to the function of promoting blood and qi circulation and eliminating stasis in HHRY pill. HHRY pill consisted of 26 traditional medicines. Flos Carthami (Honghua) and Stigma Croci (Xihonghua) are the JUN medicine, the most important components, in the HHRY formula. They have good effect in blood activating, pain as well as stasis relieving. Modern researches reported that the main components of Flos Carthami and Stigma Croci, safflower yellow and crocin, could improve microcirculation and anti-inflammation, which would contribute to the treatment of PID [7-9]. When compatible with qi- promoting herbs Racemose Jurinea (Zangmuxiang) [10], Lignum Dalbergiae Odoriferae (Jiangxiang) [11], etc. the effect of HHRY pill is enhanced in promoting blood and qi circulation as well as relieving pain. An pharmacology research also reported that HHRY pill synergistically alleviated the inflammation and adhesion of pelvic by regulating the expression of inflammatory cytokines interleukin-2 (IL-2) and tumor necrosis factor (TNF-α) as well as intercellular adhesion molecular (ICAM-1) in endometrium[4,5]. Therefore, this study speculated the effect of HHRY pill for relieving CCP caused by PID. 261 subjects from 9 hospitals in different areas of China participated to take three menstrual cycle treatment and helped to prove this effect of HHRY pill.
Conclusion
Though the sample size was statistically sufficient and the intervention time was three months, the efficiency of HHRY pill for relieving CCP induced by PID only evaluated subjective and symptoms assessments. Biochemical indicators, for example, IL-2, TNF-α and so on, are supposed to be measured, which will further reflect the effectiveness of HHRY pill. In summary, a clinical trial with sufficient sample and study period proves the effectiveness and safe of HHRY pill in alleviating pain and related symptoms of CCP. The study also indicated HHRY pill would reduce patients’ psychological burden and enhance the quality of life during the treatment period. However, more assessments of biochemical indicators are needed to investigate the effect.
Trial registration
ClinicalTrials.gov ID: ChiCTR-IPR-15006945.
Conflicts of Interest
None of authors declares that they have no conflicts of interest.
Acknowledgements
The authors thank other clinical researchers who assisted this trial and Gannanfoge Tibetan Medicine Company Limited which offered the HHRY pills.
Role of the Funding Source
This post-marketing clinical trial was sponsored by Gannanfoge Tibetan Medicine Company Limited, which produced HHRY pill. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- Bulletins-Gynecology. Chronic pelvic pain. Obstet Gynecol 2004; 103: 589-605.
- Krywko LKJDM. Pelvic Inflammatory Disease (PID). 2019.
- ChengY, Guo T, Yuan Y, Jin Y, Xu N. Acupuncture for chronic pelvic inflammatory disease: A systematic review protocol. Medicine (Baltimore) 2018; 97: e0225.
- Qi, Z. Treatment of Chronic Pelvic Inflammation by Honghua Ruyi Pill in Rats. Chinese Journal of Experimental Traditional Medical Formulae 2011.
- Chaojin XOQ. Curative Effect of Honghua ruyi Pill on Chronic Pain Caused by Pelvic Inflammatory Disease and Its Possible Mechanism. World Chinese Medicine 2016.
- Dai Zhang ZL, Lin H, Liao Q. Effectiveness of Honghua Ruyi Wan combined with antibiotics for relief of pelvic inflammatory disease pain in women. Biomed Res 2017; 28: 4665-4670.
- YANG Yu, TONG Jie. Analgesic effect of safflomin A and its mechanism. Cent South Pharm 2019.
- Tu Y, Xue Y, Guo D, Sun L, Guo M. Carthami flos: a review of its ethnopharmacology, pharmacology and clinical applications. Revista Brasileira de Farmacognosia 2015; 25: 553-566.
- Boskabady MH, Farkhondeh T. Antiinflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L. and its main constituents. Phytother Res 2016; 30: 1072-1094.
- Das A, Shakya A, Ghosh SK, Bhat HR. A review of phytochemical and pharmacological studies of inula species. Curr Bioact Compd 2020; 16: 557-567.
- Zhao X, Wang C, Meng H, Yang M, Wei J. Dalbergia odorifera: A review of its traditional uses, phytochemistry, pharmacology, and quality control. J Ethnopharma 2020; 248: 112328.