Research Article - Journal of Agricultural Science and Botany (2021) Volume 5, Issue 6
Running title: Endophyte Co-treatment on Persian Oak Tree Evaluation of Endophyte Co-treatment on Persian Oak Tree (Quercus brant ii) Issue Type: Regular Issue
Yousef Azarakhsh*
Department of Microbiology, Razi University, Kermanshah, Iran.
- *Corresponding Author:
- Yousef Azarakhsh
Department of MicroBiology Razi University, Kermanshah Iran
E-mail: y.azarakhsh@razi.ac.ir
Accepted on May 17, 2021
Citation: Azarakhsh Y. Evaluation of Endophyte Co-treatment on Persian Oak Tree (Quercus brant ii). J Agric Sci Bot. 2021;5(6): 057.
Abstract
The Zagros forests are one of the typical crucial ecosystems placed in western Iran. A remarkable area of these forests situated in Kermanshah province. Due to human manipulation, ecological and climate change are deteriorating. In this investigation, microbial isolation and treatments were offered to evaluate their effects on oak growth.
Samples collected from Quercus brant ii of Kermanshah regions, Iran, and were cultivated on ISP2 and NA medium, kept at 28°C for one month. The Microscopic identification and phosphate solubilization assay for primary and nitrogen fixation, hydrogen cyanide, protease, and phytohormone production performed. Lastly, Seedlings with selected isolate treated and growth and survival parameters measured.
All strains were able to produce auxin and gibberellin in different values. All of the isolates able to solubilize phosphate. The outcomes of nitrogen fixation ability, protease, and siderophore production diverged among strains. Picked isolates exhibited hormone production properties as well as biocontrol influences according to tests. Co-treatment of Streptomyces sp. Qb2-Pseudomonas sp. Qb, Streptomyces sp. Qb2-Bacillus sp. Qb and Streptomyces sp. Qb2- Pseudomonas sp. Qb had a notable effect on seedling growth parameters (P <0 •001), oak species survival (P <0 •001), and improved significantly the longitudinal growth, diameter, and dry weight percent of seedlings (P <0 •005) Respectively. Streptomyces sp. Qb2 and Bacillus sp. Qb strains had the highest and lowest inhibition effects on bacterial plant pathogen, Pseudomonas syringae. Streptomyces sp. Qb2 strains significantly promote root formation. To our understanding, this is the first record of Streptomyces endophyte isolation from Iranian oak trees with biocontrol and growthinducing impacts. It is also proposed to apply the co-treatment plan to increase biocontrol effects and growth induction.
Keywords
Iran, Oak, Endophytic bacteria, Co-treatment, Streptomyces, Quercus brant ii
Introduction
Plant Species The Zagros forests are one of the typical crucial ecosystems placed in western Iran. A remarkable area of these forests situated in Kermanshah province. The foremost tree species in the area is Quercus brant ii (Qb), with 70% of the Zagros forest species being oak [1]. Quercus is one of the most recognized genera of Fagaceae in courses of species, geographic distribution, environmental rank, and ecological, and economic state. Due to community growth, fuel consumption, livestock feed, land expansion, road building, electricity, and telephone lines, planned development, industrial projects, and plant pathogens, these forests are on the deterioration [2]. Across the last three decades, the presence of endophytic microorganisms has been shown to serve their host [3]. Endophytic bacteria improve plant growth, ability to solubilize phosphate, and contribute assimilable nitrogen to plants. They have active cooperation [4] or that these symbiotic microorganisms enhance the host's ability to adapt and survive and to grow.
The name endophyte introduces the permanence of microorganisms inside the crowded plant tissues outdoors, having adverse impacts on the organism plant [5]. The bacteria are farmers of growth-promoting metabolites, insect and germ repellents, antimicrobials facing plant pathogens, supporters in pressure states, and multiple more extended [6]. They additionally maintain the potential to deliver novel auxiliary metabolites, which can employ in pharmaceutical, agricultural, and other manufacturers.
These bacteria colonize every similar environmental niche in plants as plant pathogens. They have extensively recognized tools of biocontrol motion before-mentioned as they fight for ecological niche or substrate, making of inhibitory compounds, and induced systemic confidence in the host toward a full spectrum of pathogens [7]. Also, the synergies between plants and endophytic bacteria might help plants to settle in ecosystem recovery methods. Individual plant species might be a host for one or more further endophytic bacterial species. The presence of endophytic bacterial species and their population density is genuinely variable depending on the bacterial varieties, hostess genotypes, and environmental states [8].
Thus, there is a developing interest in bioprospecting of endophytic microbial communities from various ecosystems. Among which actinobacteria required because of the production of different biologically active materials of commercial value. Rhizobacteria, bacteria, epiphytes, and endophytes are also important. The role of colonizing bacteria in plant tissues has studied [9]. Endophytes can affect germination, plant propagation under different conditions, promote plant growth, or limit infection of plant pathogens [10]. Other gains
of endophytic symbiont bacteria such as nitrogen fixation, immune system inducers and enhancers of available minerals and reduced ethylene production lead to survival and adaptation of the host [11]. It also promotes disease resistance and adaptive power by producing a wide range of secondary metabolites [12] and stimulates plant host growth.
The inherent symbiotic bacteria isolated from forest trees include Pseudomonas, Bacillus, Acinetobacter, Actinobacteria, Sphingomonas, and Enterobacteriaceae [13]. Investigations in the area of endophyte actinomycetes from different environments are insufficient. The only study in Iran focuses on gram-negative endophytes. (Marivan) Forests have investigated [14]. Nevertheless, the search for gram-positive actinomycetes has not conducted. Furthermore, only the study of actinomycetes affecting physiological and pathological processes [15] has investigated.
The management, restoration, and regeneration of forests using the biological capabilities of active microbial metabolites have so far been neglected in Iran, notably in the western Zagros and Kermanshah provinces, to control the deterioration of oak trees. Two areas, which have very positive economic and environmental potential, are biocontrol and biodegradation, research. This study aimed to evaluate the effects of oak endophytes on growth parameters and survival. Which, if the objectives of this study succeed, choose the isolates as biocontrol and biodegradation.
Material and Methods
Sampling locations
Zagros are oakforests that dominate between 1000 and 2000 m elevation and account for nearly 40% of Iran's covers (Haidari and Rezaei 2013). This study was carried out in five natural forest stands dominated by Persian oak (Qb.) located in the west of Iran, including: Dalahoo 1 (34° 23' 28 N, 46° 03' 20 E), Kerend (34° 20' 19 N, 46° 07' 42 E), Dalahoo2 (34° 10' 42 N, 45° 22' 15 E), Islamabad (34° 10' 43 N, 46° 39' 01 E).
Sampling
Rhizosphere soil of oak trees (Q. brant ii) sampled at different stations in the spring, and 50 g of samples transferred to the laboratory (4°C). Homogeneous soil samples counted until the experiment. Plant elements such as leaves, stem, roots prepared freshly or saved at 4°C until isolation in 24 h.
Cultivation and purification of bacteria
Samples treated initially; the examples rinsed with distilled water. Leaf sample surfaces decontaminated with 5% sodium hypochlorite for 5 min and three times. 50 μl of the final wash was cultured NA1medium to assay sterilization. The tissue sections were then cut and suspended in 5ml distilled water for 30 min and dried on autoclaved paper filters [16]. These explants cleaned in running tap water to murder adhered epiphytes, soil particles on the outside. Then sterilization by ethanol for 2–4 min performed [17]. Treatment methods including ((NaClO3) 5% (Na2S2O3), 2.5%, ethanol 75% and (NaHCO3) 10%) were used to disinfect and repress fungal extension. The force of cleaning chemicals depends on the permeability of the sample. Contrarily, the inside tissues disinfect [18]. All the explants were lastly washed with pure distilled water, divided into small scraps (for steam or roots 1 cm and leaves1 cm2) and suspension injected on suitable agar medium.
After pre-treatment, 10 g of the soil sample was well homogenized, dried, and mixed with saline, and the Dilution serial was prepared (10-1 to 10-5). A loop of the desired dilution of each sample was cultured on the ISP2 medium2 (g L−1): (Yeast extract (Difco)4.0 g, Malt extract (Difco) 10.0 g, Dextrose (Difco) 4.0 g, Agar 20.0 g 1000.0 ml Distilled water pH 7.2).
The media were enriched using nystatin (50 μg /ml) to suppress fungal growth and stored at 30°C for one month. Single colonies with unique actinomycete morphology arising out from the plant mass are separate. The purified cultures of the isolates collected by streaking on fresh media plates. Colonies with different appearance and sporulation determined for subsequent stages and microscopic and biochemical identification. Actinomycetes isolated by plating serial dilutions on the ISP2 medium. After one h of incubation at 90°C, purified cultures were taken from picked colonies for renewed subculturing on casein agar. The appearance characteristics were identified based on the color of the spore chain, surface mycelium color, soluble pigment color, and spore morphology [15]. Then, for the identification of strains gram and biochemical tests done.
Phosphate dissolution
The ability of the isolates to dissolve phosphate was calculated using the method. Petri dishes containing the Picosawa34 The culture medium was treated with 2 μl of suspension and incubated at 28°C for seven days. The generation of transparent halo near the colony indicates the dissolution of phosphate. Screening of isolates for MPS5 the activity performed on (HAP)6 At 5 g /L as a single phosphate source . Following seven days of incubation at 30°C, the plates examined for the presence of colonies developing apparent haloes (zone of solubilization). Spores of the actinomycetes isolates showing the largest halo zones were stored in 20% (w/v) sterile glycerol at −80°C until further use.
Hormonal Assessment (Indole Acetic Acid (IAA) and Gibberellic Acid (GA) production)
For this bacterial assay, isolates were cultured in 25 ml broth medium with 2% L-tryptophan and incubated at 28°C for one week. Then The media centrifuged for 10 min (2100 rpm), and 1 ml of supernatant added to a 2 ml tube containing Salkowski's solution. The resulting mixture incubated in the dark for 45 min. The red color indicates the production of the endothelial hormone. A spectrophotometer read the adsorbed combination at 530 nm. The concentration of indoleacetic acid produced by the isolates was calculated using the curve plotted by standard indole acetic acid at dilutions of 0, 3, 6, 12, 25, 50, 125 and 250 μg /ml [19]. In another measurement, the Gibberellic
production rate was calculated using the Holbrook method [20]. In summary, the 24-hour culture of the bacteria was inoculated in Jenson broth medium7Furthermore, incubated at 200°C for 24 hours. After centrifugation at 1850 for 2 min, the resulting supernatant was filtered and acidified with second hydrochloric acid to pH = 1-2 and concentrated and purified by ethyl acetate volume three times. The resulting gibberellic acid was separated again by the gradual addition of 15.20 and 10 ml of phosphate buffer at pH = 7.2. Each extract was added to a 100 ml flask and read at 254 nm. Gibberellic acid concentration of each isolate was determined using Gibberellic acid standard at levels of 0, 3, 6, 12, 25, 50, 125, and 250 μg /ml and curve plotting.
Plant growth-promoting potential of endophytic bacteria
Nitrogen fixation
Nitrogen-free semisolid malate1 Medium made with or without ammonium chloride as a nitrogen source. After sterilization with an autoclave for 15 min, pre-plating the vitamin solution containing biotin and peroxide added. The isolates were inoculated and incubated for seven days at 28°C to observe incubation growth [21] and [22].
Protease Production
Protease production performed according to the method of Sgroy, Petri dishes inoculated with 10 μl of bacterial isolates in Schim Milk Agar2 Medium and incubated at 28°C for 4 days. The formation of a bright halo around the bacterial colonies indicates the presence of an alkaline protease enzyme [22].
Production of hydrogen cyanide
All isolates were screened to produce hydrogen cyanide by the method of Alstrom and Burns (1989) [23]. Fifty microliters of each bacterial suspension were cultured on the NA medium containing Whitman paper impregnated with the picric acid solution inside the plate. The culture plates were blocked with parafilm and incubated at 2828°C for seven days. The change of yellow to orange or red indicates the production of hydrogen cyanide by the desired isolate.
Biocontrol activity of endophytic bacteria against pathogen
Bacterial plant pathogens, Pseudomonas syringae PV. Syringe strain previously identified as a broad- and narrow-host range bacteria, respectively, were chosen as indicators. 300 μl of suspension (2 × 108 3CFU /ml) from bacterial pathogen Pseudomonas syringa was spread on a nutrient agar plate and kept at room temperature for 5 min. Then, sterile paper discs preimmersed in the suspension of endophytic bacteria (concentration of about 108 CFU/ml) spotted on the pathogeninoculated plates. Plates incubated at 28°C for 48–72 h and the width of the inhibition zones surveyed. Sterile water located in the plates with the pathogen was done as a control [24] The operation was duplicated twice with four replications.
1 DL-malic acid: 5.0 g, K2HPO4: 0.5 g, MgSO4 • 7H2O: 0.2 g, NaCl: 0.1 g, CaCl2 • 2H2O: 0.02 g. Micronutrient solution: 2 ml,
7 Ingredients g L-1: Sucrose 20.000, Dipotassium phosphate 1.000, Magnesium sulfate 0.500, Sodium chloride 0.500, Ferrous sulphate 0.100, Sodium molybdate 0.005 Calcium carbonate 2.000.
Bromthymol blue solution (0.5% in 0.2N KOH): 2 ml, Fe (III) EDTA (1.64%): 4.0 ml, Vitamin solution: 1.0 ml, Distilled water: 1.0 L, Adjust pH to 6.8, For semisolid medium, add 0.5 g of agar; for solid medium, add 15 g of agar. Autoclave at 121°C for 15 min. Micronutrient solution: CuSO4 • 5H2O: 0.4 g, ZnSO4
• 7H2O: 0.12 g, H3BO3: 1.4 g, Na2MoO4 • 2H2O: 1.0 g, MnSO4
• H2O: 1.5 g, Distilled water: 1.0 L, Vitamin solution: Biotin: 10 mg, Pyridoxol HCL: 20 mg, Distilled water: 0.1 L.
228g Skim milk powder, 5g Casein enzymic, 2.5g hydrolysate Yeast extract, 1 g Dextrose, 15g Agar, Final pH (at 25°C) 3colony-forming unit
Inoculation in seedlings
Four groups of annual seedlings in pots containing soil of Kermanshah oak forests by selected bacterial samples from the initial screening of Streptomyces sp., Pseudomonas sp. Bacillus sp. were inoculated. Seedlings inoculated with 15 ml of CFU/ ml (3 × 108) bacterial suspension (prepared using McFarland Standard No. 1). Also, Negative control samples inoculated with 0.1% saline [25]. Seedlings were harvested three months after the bacteria were fixed and inoculated. Various vegetative parameters including fresh weight, leaf dry weight, stem, the root measured, and leaves measured after imaging with image j software. Seedling height, root length was measured. Finally, the ratio of fresh weight to shoot weight calculated. The current biomass weight (root, shoot, and leaf weight) was also calculated [26].
Data Analysis
Data analysis was performed using Graph Pad Instate prism 8.1, and a comparison of means made with LSD test at the 1% level. By ANOVA-Two-Way test and Dunnett's test. Results were analyzed with 95% confidence level (P <0 · 05, P <0 · 001, P <0 · 0001).
Results and Discussion
Identification of endophytic bacterial isolates
Based on preliminary screening tests of 32 microbial strains (Table 1), soluble phosphate bacteria into three genera (Streptomyces sp., Pseudomonas sp. Bacillus Sp.) selected for subsequent experiments. (Table 2) presents the results of survival variance, longitudinal, and diameter growth of oak seedlings.
| Test | Streptomyces sp.2Qb | Streptomyces sp.1Qb | Bacillus sp. Qb | Pseudomonas sp.Qb |
|---|---|---|---|---|
| Gram Test | Gram Positive | Gram Positive | Gram positive | Gram negative |
| Morphology | Filamentous | Filamentous | Bacillus | Bacillus |
| Oxidation | - | - | - | + |
| Catalase | + | + | + | + |
| Motility Test | Non-Motile | Non-Motile | Motile | Motile |
| Glucose | Variable | Fermentative | Variable | Oxidative |
| Methyl Red (MR) test | + | + | - | - |
| Voges–Proskauer test | + | + | - | - |
| Citrate | - | + | + | + |
| Nitrate | - | + | + | - |
| Indole | - | - | - | - |
Table 1. Biochemical tests to identify isolated bacteria (Mac Faddin, 1976).
Results showed that two genera of Streptomyces sp., Pseudomonas sp. and the genus Bacillus sp., were identified. Among the 40 strains collected, only four could solubilize HAP. Of these four isolates, showing the largest halo zones and selected for further investigations [27].
Measuring of PGP1
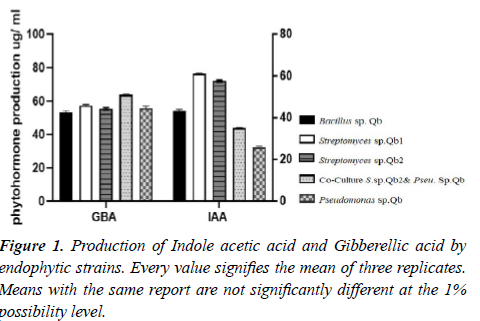
As can be seen from the details in Table 2, the biosynthesis of indole acetic and gibberellic acid hormones was different among the isolates. Among the isolates were treated with Streptomyces sp.2Q and Pseudomonas sp. Qb accounted for the highest production of 84.11 and 61.14 μg /ml, respectively. Streptomyces sp.1Qb and Bacillus sp. Qb Strains subjected to the creation of these two hormones. Streptomyces sp.1Qb isolate treatment was associated with the highest production of gibberellic acid (76.34 μg /ml) (Table 2, Figure 1). All isolates were able to dissolve phosphate. Other indicators of growth promotion activity shown in (Table 2).
1Plant growth-promoting
| Strain | N.f | N.f | Growth inhibition of P.syringae |
||||
|---|---|---|---|---|---|---|---|
| Pseudomonas sp.Qb | 82 ± 1.6a | 56.22± 1.61a | 32.11 | + | - | - | + |
| Bacillus sp. Qb | 72 ± 1.8a | 52.21± 1.91a | 54.22 | - | - | - | _ |
| Streptomyces sp.1Qb | 93 ± 1.9a | 58.12 ± 2.31a | 76.34 | + | + | + | + |
| Streptomyces sp.2Qb | 95 ± 1.9a | 44.34± 2.01a | 58.12 | + | + | + | + |
| Co-culture mixture of Streptomyces sp.2Qb & Pseudomonas sp.Qb | 105 ± 1.1a | 64.16± 1.72a | 44.23 | + | + | + | + |
Table 2. Plant growth potential properties of Endophytic strains oak trees and co-treatment.
Measure the biocontrol activity of the isolates
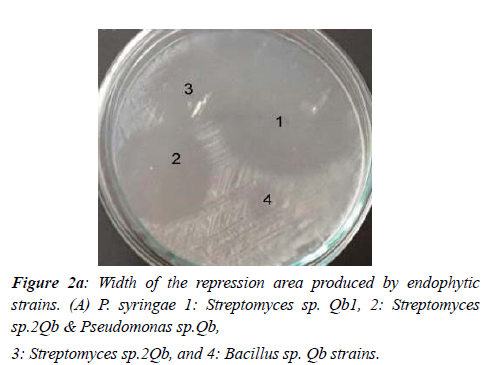
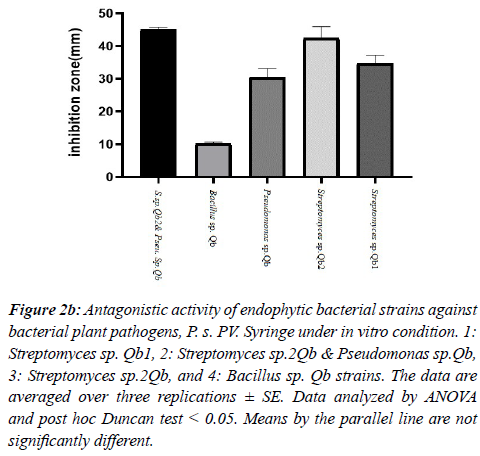
Among the tested strains of Streptomyces sp.1Qb with an average inhibitory zone diameter of 11.1 mm against P. syringae PV. The syringe has the highest inhibitory effect on this plant pathogen. Bacillus strain had no inhibitory effect on pathogen growth. Both Streptomyces sp.2Qb & Pseudomonas sp. Qb and Streptomyces sp.2Qb vessels were next in growth with 9- and 8-mm diameter inhibition zone, respectively (Figure 2 a, b).
Figure 2b: Antagonistic activity of endophytic bacterial strains against bacterial plant pathogens, P. s. PV. Syringe under in vitro condition. 1: Streptomyces sp. Qb1, 2: Streptomyces sp.2Qb & Pseudomonas sp.Qb, 3: Streptomyces sp.2Qb, and 4: Bacillus sp. Qb strains. The data are averaged over three replications ± SE. Data analyzed by ANOVA and post hoc Duncan test < 0.05. Means by the parallel line are not significantly different.
Effects of Biomass Treatment
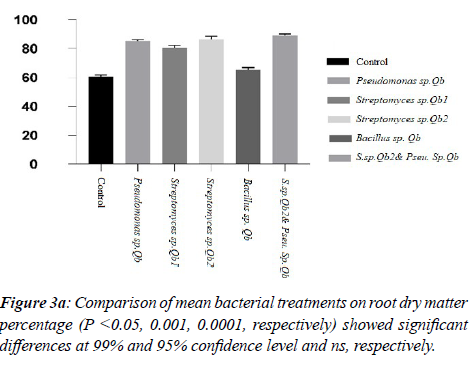
Among the selected isolates, co-treatment of the Bacillus and Streptomyces group had significant effects on seedlings viability and strains of Bacillus sp. Qb, Streptomyces sp. Qb2, Streptomyces sp. Qb2 were ranked respectively. The results of Pseudomonas isolate on seedling viability were not significant (Table 4). 3.5 Effects on diameter and length of oak seedlings
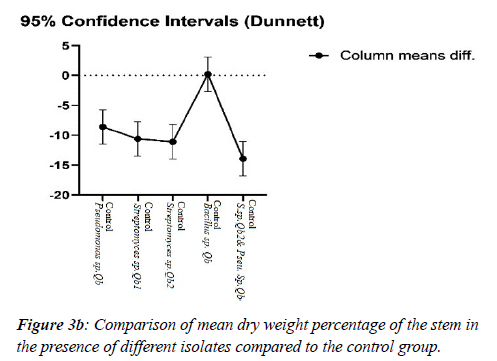
According to the data, the effects of on seedling diameter by variance test and Dunnett's experiment showed that co-treatment with Bacillus-Streptomyces isolates had the highest mean diameter increase among other isolates (Bacillus, Streptomyces (P <0.001), and Pseudomonas isolates had no significant effect on average diameter of seedling growth (Figure 3a, b; Table 3a,b; Table4, Table 5a, b).
| Dunnett's test | P-value | Conf. |
|---|---|---|
| Control & Pseudomonas sp. Qb | 0.9986 | Ns |
| Control &. Streptomyces sp. Qb1 | 0.0025 | ** |
| Control & Streptomyces sp. Qb2 | 0.0018 | ** |
| Control & Bacillus sp. Qb | 0.0007 | *** |
| Control & Co-treat Bacillus Sp.+ Streptomyces <0.0001 sp. | **** | |
Table 3: aNumbers given are the average of triplicate experiments ± SD. (P <0.05 *, ** P <0.001)
Table 3a. Comparison of mean diameter biomass of oak stems. Tab. 3b: Comparison of mean diameter biomass of oak seedlings.
| P <0.05, 0.001, 0.0001, respectively, were signific and ns respectively not significant. 3b: | Count at 99% and 95% c | Confidence level |
|---|---|---|
| P-value | Conf. | |
| Control vs. Pseudomonas sp. Qb | 0.2038 | ns |
| Control vs. Streptomyces sp. Qb1 | 0.0444 | * |
| Control vs. Streptomyces sp. Qb2 | 0.0090 | ** |
| Control vs. Bacillus sp. Qb | 0.0057 | ** |
Table 3b: P <0.05, P <0.0001, respectively, were significant at 99% and 95% confidence level and ns respectively not significant.
| Bacterial treatment | Dry weight percent | ||
|---|---|---|---|
| Pseudomonas sp.Qb | Root | Stem | Leaf |
| 57±1/0 | 79.2±/3.1 | 57.6±/1.5 | |
| Bacillus sp. Qb | 65.4± 47/ 0 | 70.6±/2.6 | 43.1±/2.4 |
| Streptomyces sp.1Qb | 80.6 ± 1/0 | 80.2±3.1 | 59.4±/1.49 |
| Streptomyces sp.2Qb | 86.13 ± 3/0 | 81.7 ±3.4 | 60.2±/3.1 |
| Co-culture mixture of | 89.27 ± 2/0 | 84.2±3.2 | 63.9±/4.5 |
| Streptomyces sp.2Qb & Pseudomonas sp.Qb Control | 60.6 | 70.9±2.3 | 42.9±3.55 |
Table 4. Results of species treatment based on root, stem, and leaf dry matter of oak.
| Dunnett's test | P-value | Conf. |
|---|---|---|
| Control vs. Pseudomonas sp. Qb | 0.9997 | No |
| Control vs. Streptomyces sp. Qb1 | 0.3273 | No |
| Control vs. Streptomyces sp. Qb2 | 0.9985 | No |
| Control vs. Bacillus sp. Qb | 0.3102 | No |
| Control vs. Co-treat Pseudomonas sp.+ Streptomyces.sp. Qb2 | 0.0015 | Yes |
Table 5a: Comparison of the mean dry weight of stem. Tab. 5b: Comparison of the mean dry weight of leaf seedlings between control and selected isolates.
| Dunnett's test | P-value | Conf. |
|---|---|---|
| Control vs. Pseudomonas sp. Qb | <0.0001 | **** |
| Control vs. Streptomyces sp. Qb1 | <0.0001 | **** |
| Control vs. Streptomyces sp. Qb2 | <0.0001 | **** |
| Control vs. Bacillus sp. Qb | 0.9997 | ns |
| Control vs. Co-treat Pseudomonas sp.+ Streptomyces.sp. Qb2 | <0.0001 | **** |
Table 5b: P <0.05, 0.001, 0.0001 Significant differences at 99% and 95% confidence level and ns respectively were not significant, respectively.
Measuring seedling growth
Table 4 shows the concordance of the Streptomyces sp.2Qb &Pseudomonas sp. Qb had a significant effect on the root, stem, and leaf growth in comparison to the control (Table 4, Figure 3a,b). Treatment with Streptomyces and Pseudomonas isolates and co-treatment also had a significant effect on root dry weight (Figure 3a; Table 5a, b). Treatment of Bacillus, Pseudomonas, and Streptomyces isolates alone did not have a substantial impact on leaf growth parameter, but also co-treatments of Streptomyces sp.2Q & Pseudomonas sp. Q on leaf dry weight of seedlings compared with control was significant (Table 5b).
In this study, endophytic bacteria of Zagros oak trees of Kermanshah have been studied to identify their diversity, dispersal, and ability to controlling pathogens and their role in promoting host growth.
Dochhil described the plant growth enhancement and higher seed germination percentage by the employment of two Streptomyces sp. isolated from Centella Asiatica [17]. Also, these strains estimated for the production of a plant growth promoter, Indole Acetic Acid (IAA), which found in much higher concentration as 71 g/ml and 197 g/ml.
The isolates of the genus Nocardiopsis presented the highest IAA production ability among all other actinomycete genera. In the field trials conducted by ElTarabily, Actinoplanes campanulatus, Micromonospora chalcea, and Streptomyces spirals were applied individually and in aggregate to cucumber seedlings, which enhanced plant growth and yield. Gangwar also found actinobacteria, mostly Streptomyces sp., capable of producing IAA. Plant growth promoters built within the range of 9.0–38.8 mg/ml.
El-Tarabily applied endophytic Actinoplanes campanulatus, Micromonospora chance, and Streptomyces spiralis to cucumber seedlings. As it reduced seedling damping-off as well as rootand crown- rot of mature cucumber plants caused by Pythium aphanidermatum successfully, the authors suggested that these strains of endophytic actinobacteria employed as biological control agents.
The low content of free phosphorus in soil due to the formation of insoluble metallic complexes is a principal limiting factor in agriculture . Microbes in individual belonging to actinobacteria such as Streptomyces, Micrococcus, and Micromonospora, Kitasatospora, and Thermobifida, are well characterized for their capability to solubilize phosphate. The strains of Micromonospora aurantiaca and S. Griseus are reported positive for overcoming damping off caused by Pythium ultimum, and are also known for rock phosphate solubilization, indicating the importance in biological control under nutrient deficient soils. The phosphate-solubilizing activity of actinobacteria is correlated to organic acid and siderophore production.
The performance of Streptomyces strains in plant growth improvement has studied in various subjects. The inoculation of actinomycetes into the Arabidopsis rhizosphere is found to promote growth. Increase raises by actinomycetes have been correlated with root elongation, whereas for endophytic strains such effects are generally attributed to plant growth regulators production, leading to better seedling growth and protection against tissue damage. Additionally, growth promotion is also associated with siderophore production and phosphate solubilization, and nitrogen fixation. Researchers isolated endophytic actinomycetes with antibacterial activity [28], Recent studies show that the distribution of actinomycetes in different organs: leaves, roots, and stems of the same species. [29] The outcomes of this investigation reveal that the endophytes of Kermanshah oak trees are less diverse than similar studies, and Streptomyces, Pseudomonas, and Bacillus are the dominant species (Table 1).
Separation of endophytic actinobacteria depends on various constituents, which comprise host plant species, age, and type of tissue, geographic and ecological distribution, sampling season, surface sterilant, particular media, and culture requirements [18].
Dochhil [17], from the root, stem, and leaves of Centella Asiatica isolated Streptomyces sp. that produced Indole Acetic Acid (IAA) In a complex micro ecosystem. In this research, we studied the impact of growth promoting bacteria (phosphate solubilizing ability) isolated from oak trees on the growth and stability of oak species and, consequently, increase the possibility of their establishment and survival [30]. It has been published that the amount of the bacterial growth-promoting effect on the bacterium and its population, plant-bacteria composition, plant genotype, type of parameters studied, and environmental conditions depend [31].
In most cases, treatment with growth-promoting bacteria increases viability, primarily if inoculation is carried out in seedlings, and then large-rooted offspring are transferred to the target area [26]. In another study, the effect of the type of growthpromoting bacteria on growth rate based on dry weight gain has emphasized [32].
This research is in agreement with the results of this study on the effect of selected isolates on dry weight growth (Table 5 & Figure 3). Effect of Inoculation on height and collar diameter increase by growth-promoting bacteria have reported in previous studies. The findings of this study regarding the effect of growth-promoting bacteria on height are consistent with the results of others regarding the direct relationship between growth with genus and species of bacteria[31]. Increased longitudinal and diameter growths of Eucalyptus species have been reported using Pseudomonas and Bacillus inoculation [33]. The effect of Streptomyces, Pseudomonas, and Bacillus isolates on different parameters of oak in the present study is significant and is consistent with other researchers. Based on the results of various studies showing the effect of inoculation on longitudinal and diameter extension, the outcomes of the instant research prove the role of bacterial endophyte growth (plant hormone production and root uptake) (Table 2 and Figure 2 a, b).
According to the events of the instant study, in comparison with other similar studies [34] and [35], the role of Pseudomonas and Bacillus in the growth effect of filamentous actinobacteria in growth promotion is determined, especially in co-treatment conditions of Streptomyces and Bacillus isolates. The viability of oak seedlings was remarkable (Table 4 and Figure 3). Overall, it seems that the role of Pseudomonas and Streptomyces isolates in terms of growth parameters and production of different hormones on oak species of Dalahoo station. Selected isolates either in a single treatment or in co-treatment as biocontrol agents (Streptomyces sp. Qb1) and growth stimulator (Streptomyces sp. Qb2 & Pseudomonas sp. Qb) Kermanshah oak trees and control their deterioration in conservation programs and the restoration of such oak habitats are introduced (Figure 1, Table 4& 5a, b).
Conclusions
The result of the present study helps us to manage and control of plant pathogens and reforestation. Especially when different pathogens cause oak degradation, biological control is one of the most sustainable ways to combat the decline of Kermanshah oak trees. Description of selected Streptomyces isolates of oak trees of Zagros region and characterization of their metabolites recommended.
Funding
No other competing interests to be declared.
Acknowledgments
This research is done in collaboration with Soren Lab, and thanks for the sincere cooperation of its management.
2International Streptomyces Project 2 medium
341, (g l−1: glucose10; Ca3(PO4)2, 5; (NH4)2SO4, 0.5; NaCl, 0.2; MgSO4.7H2O, 0.1; KCl, 0.2; yeast extract, 0.5; MnSO4.2H2O, 0.002; and FeSO4.7H2O, 0.002.
5Mineral phosphate-solubilizing
7Ingredients g L-1: Sucrose 20.000, Dipotassium phosphate 1.000, Magnesium sulfate 0.500, Sodium chloride 0.500, Ferrous sulphate 0.100, Sodium molybdate 0.005 Calcium carbonate 2.000.
References
- Pourhashemi M, Mohajer MRM, Zobeiri M, et al. Identification of forest vegetation units in support of government management objectives in Zagros forests, Iran. Scand. JFor Res. 2004;19(S4):72-77.
- Abedini R, Salooki MK, and Ghasemian SJCERB. Modeling and simulation of condensed sulfur in catalytic beds of the Claus process: rapid estimation. Chem Eng Res Bull.2010;14(2):110-14.
- Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomater Res.2015;73: 254-71.
- Rosenblueth M, Martínez-Romero EJMpm. Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact.2006;19(8):827-37.
- Schulz B, Boyle C. What are endophytes? Microbial root endophytes.2006;1-13.
- Ryan RP, Germaine K, Franks A, et al. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett.2008;278(1):1-9.
- Compant SB, Duffy J, Nowak C, et al. Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and prospects. Appl Environ Microbiol. 2005;71(9):4951-59.
- Chebotar VN, Malfanova A, Shcherbakov G, et al. Endophytic bacteria in microbial preparations that improve plant development. Appl Biochem Micro. 2015;51(3):271- 77.
- Bulgarelli DK, Schlaeppi S, Spaepen EVL, et al. Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol. 2013;64:807-38.
- Hurek, Thomas, and Barbara Reinhold-Hurek. Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J Biotechnol. 2003;106:169-78.
- Bacon C, Hinton D, and Hinton Jr AJJoam. Growth- inhibiting effects of concentrations of fusaric acid on the growth of Bacillus mojavensis and other biocontrol Bacillus species. J Appl Microbiol. 2006; 100(1):185-94.
- Strobel G, Daisy B, Castillo U, et al. Natural products from endophytic microorganisms. J Nat Prod. 2004;67(2):268- 247.
- Pirttilä AM, Frank AC . Endophytes of forest trees. 2011.
- Tashi Oshnoei F, Harighi B, Abdollahzadeh JJFp . Isolation and identification of endophytic bacteria with plant-growth- promoting and biocontrol potential from oak trees. Pathol. e.2017;47(5).
- Azarakhsh Y, Mohammadipanah F, NassiriS, et al. Isolation and screening of proangiogenic and antiangiogenic metabolites producing rare actinobacteria from the soil. J Appl Microbiol .2017;122(6):1595-602.
- Doust NH, Akbarinia M, Safaie N, et al. Community analysis of Persian oak fungal microbiome under dust storm conditions. Fungal Ecol. 2017;29:1-9.
- Dochhil H, Dkhar MS, Barman D. Seed germination enhancing the activity of endophytic Streptomyces isolated from indigenous ethnomedicinal plant Centella Asiatica. Int J Pharm Biol Sci. 2013;4(1):256-62.
- Hallmann J, Berg G, Schulz B. Isolation procedures for endophytic microorganisms. Microbial r endo. 2006;299- 319.
- Rahman A, Sitepu IR, Tang SY, et al. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci Biotech Bioch. 2010;74(11): 2202-08.
- Holbrook AA, Edge W, and Bailey F. Spectrophotometric method for determination of gibberellic acid, ACS Publications.1961.
- Boddey RM, De Oliveira O, Urquiaga S, et al. Biological nitrogen fixation associated with sugar cane and rice: contributions and prospects for improvement. Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems.1995;195-209.
- Sgroy V, Cassán F, Masciarelli O,et al. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated with the halophyte Prosopis strombulifera. Appl Microbiol Biot. 2009;85(2):371-81.
- Alström S, Burns RGJB, and Soils FO. Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biol Fertil Soil.1989;7(3):232-38.
- Aliye N, Fininsa C, Hiskias YJBC. Evaluation of rhizosphere bacterial antagonists for their potential to protect potato (Solanum tuberosum) against bacterial wilt (Ralstonia solanacearum).Biol Control. 2008;47(3):282-88.
- Mac Faddin JF. Biochemical tests for identification of medical bacteria.1976.
- Probanza AJ, Mateos JL, Garcia B, et al. Effects of inoculation with PGPR Bacillus and Pisolithus tinctorius on Pinus pinea L. growth, bacterial rhizosphere colonization, and mycorrhizal infection. Microb Ecol.2001;41(2):140-48.
- Seshadri S, Ignacimuthu S. Variations in heterotrophic and phosphate solubilizing bacteria from the Chennai, southeast coast of India. 2002.
- Qin S, Xing K, Jiang JH, et al. Biodiversity, bioactive natural products, and biotechnological potential of plant- associated endophytic actinobacteria. Appl Microbiol Biot 2011;89(3):457-73.
- Tian X, Cao L, Tan H, et al. Diversity of cultivated and uncultivated actinobacterial endophytes in the stems and roots of rice. Microb Ecol. 2007;53(4):700-07.
- Goteti PK, Emmanuel LDA, Desai S, et al. Prospective zinc solubilizing bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int J Microbiol. 2013.
- Aslantas R, Cakmakçi R, Sahin FJSh. Effect of plant growth-promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci Hortic. 2007;111(4): 371-77.
- Garcia JAL, Domenech J, Camacho Ma, et al. Growth of forest plants (pine and holm-oak) inoculated with rhizobacteria: relationship with microbial community structure and biological activity of its rhizosphere. EEB.2004;52(3):239-51.
- Mafia RG, Alfenas AC, Ferreira EM, et al. Mounteer. Root colonization and interaction among growth- promoting rhizobacteria isolates and eucalypts species. Rev arvore.2009;33(1):1-9.
- Jin CW, Ye YQ and Zheng S, et al. An underground tale: contribution of microbial activity to plant iron acquisition via ecological processes. Ann Bot. 2013;113(1): 7-18.
- Vurukonda S, Giovanardi D, Stefani E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. As Endophytes. Int J Mol Sci.2018.