Research Article - Biomedical Research (2017) Volume 28, Issue 11
RP-HPLC determination of chlorogenic acid in Lycium barbarum L. extract and its protective effect on retinal ganglion cells
Qi Zhao*, Xiao-Xuan Wu, Jun Zhou and Xiao Wang
Department of Ophthalmology, the Second Hospital of Dalian Medical University, Dalian, Liaoning Province, PR China
- *Corresponding Author:
- Qi Zhao
Department of Ophthalmology
The Second Hospital of Dalian Medical University, PR China
Accepted on March 25, 2017
Abstract
To determine the chlorogenic acid content in Lycium barbarum L. by HPLC (Reversed Phase Highperformance Liquid Chromatography); and to observe the protective effect of Lycium barbarum L. extract on Retinal Ganglion Cells (RGCs) in rats with ocular hypertension. A rat model of chronic ocular hypertension was established using healthy adult rats. The rats were randomized into the model group and the treatment group. The effects of Lycium barbarum L. extract on Intraocular Pressure (IOP), multifocal Electroretinogram (mfERG), apoptosis of RGCs and retinal Nitric Oxide (NO) content of rats with ocular hypertension were observed. The average recovery of chlorogenic acid was 100.1%, RSD=0.9%. Chlorogenic acid content in Lycium barbarum L. extract was 40.71 mg/g. Lycium barbarum L. extract could effectively prevent the elevation of IOP; improve the rat mfERG; significantly lower the retinal NO content in rats; and reduce the apoptosis of RGCs. Lycium barbarum L. extract has a protective effect on RGCs in rats with ocular hypertension. The proposed HPLC method is simple, accurate and reproducible, which can be used as the mass control standard of Lycium barbarum L. extract.
Keywords
Lycium barbarum L., Chlorogenic acid, RP-HPLC, Retinal ganglion cell, Multifocal electroretinogram, Nitric oxide
Introduction
Lycium barbarum L. is a Solanaceae plant, whose medicinal part is the dried ripe fruit. It is neutral in nature and sweet in taste, which enters the liver and kidney meridians and has the functions of nourishing liver and kidney and improving visual acuity. Modern pharmacological studies have shown that Lycium barbarum L. has antitumor, hypotensive, anti-aging, hypolipidemic and nervous system regulating activities [1-3]. Lycium barbarum L. has been gaining increasing academic attention as a valuable medicinal and edible resource. It mainly contains polysaccharides, betaine, flavonoids and phenolic acids [4-6]. Among them, phenolic acids have antioxidant, free radical scavenging, antitumor, immunoenhancing, antiinflammatory, antibacterial effects and retinal protection [7-10], which are thus one of the material bases for bioactivity of Lycium barbarum L.
The exact mechanism of glaucomatous optic nerve injury is unclear yet, but the final outcome is always apoptosis of RGCs [11,12]. At present, the optic nerve protective therapies targeting blockage or delay of primary and/or secondary RGC injury and protection of RGGs have been one of the focuses of glaucoma treatment. Chinese medicine has advantages in this respect. Many Chinese drugs have such actions, among which Lycium barbarum L. extract is a representative. In this study, we observed the protective effect of Lycium barbarum L. extract on RGCs in rats with persistent ocular hypertension and made qualitative and quantitative analyses on chlorogenic acid in the extract by RP-HPLC technique. The method established herein was simple, rapid, accurate and reliable, which could preferably control the mass of Lycium barbarum L. extract.
Experiment
Instruments and reagents
Agilent 1260 HPLC system; Lycium barbarum L. (origin: Qinghai), identified by the associate professor Huang of the Tianjin University of Traditional Chinese Medicine as the dried mature fruit of Solanaceae plant Lycium barbarum L.; chlorogenic acid reference (National Institutes for Food and Drug Control, batch number: 20161512-3301); HPLC grade acetonitrile; purified water; and all other reagents were of analytical grade.
Animals
30 healthy adult male Wistar rats, weighing about 150 g, excluded of obvious ocular diseases, were provided by the Animal Care Facility of Nanjing Medical University (No. 1125A3712).
Methods
Preparation of plant extract: 50 g of Lycium barbarum L. medicinal material was soaked overnight in a 3-fold amount of 30% ethanol, then extracted under heat reflux for 1 h three times. After filtering while hot, the filtrates were combined, filtered and dried under reduced pressure to give the Lycium barbarum L. extract.
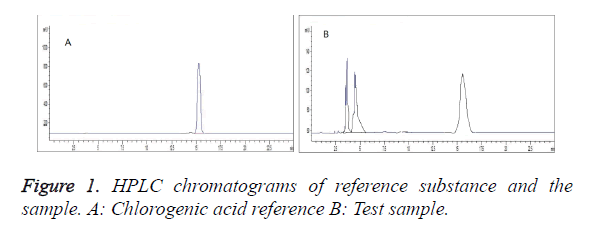
Chromatographic conditions: Column: Agilent HC-C18 (4.6 × 250 mm, 5 μm); column temperature: 30°C; mobile phase: methanol-0.1% phosphoric acid aqueous solution (11:89); detection wavelength: 327 nm; and flow rate: 1.0 ml/min. Under these conditions, chlorogenic acid can be resolved from baseline, with symmetrical peak shape and good separation. The number of theoretical plates was not less than 3,000 calculated based on the chlorogenic acid peaks (Figure 1).
Preparation of solutions: Appropriate amount of chlorogenic acid reference was accurately weighed and added with methanol to prepare a 0.50 mg/ml reference solution. About 0.5 g of Lycium barbarum L. extract was weighed dissolved in methanol, transferred to a 25 ml volumetric flask, then diluted to the mark with methanol and filtered through 0.45 μm microporous membrane to give the sample solution.
Investigation of linearity: 1, 2, 4, 6, 8 and 10 μl of reference solutions were accurately drawn and injected according to the conditions under “Chromatographic conditions”. Linear regression was performed with injection amount (μg) as the abscissa and peak area as the ordinate to derive a regression equation as Y=2.32 × 106 X-1.03 × 105 (r=0. 9997). The results showed that the chlorogenic acid content had a good linearity with the peak area within a 0.50-5.00 μg range.
Precision test: 10 μl of reference solution was accurately drawn and injected 6 repeated times. RSD of peak area was measured to be 0.92%, indicating good precision of the instrument.
Stability test: 10 μl of sample solution was accurately weighed, and injected at 0, 2, 4, 6, 8, 12 and 24 h, respectively, for determination of chlorogenic acid, RSD=0.78%. The results indicated that the sample solution was stable within 24 h from preparation.
Reproducibility test: Six aliquots of extracts from the same batch were weighed and prepared into sample solutions as per the method under “Preparation of solutions” and determined for chlorogenic acid content according to the conditions under “Chromatographic conditions”, RSD=1.5%. The experimental results indicated that the method was reproducible.
Recovery test: Nine aliquots of 0.25 g of Lycium barbarum L. extracts with known contents were accurately weighed, added sequentially with low-, medium- and high-concentrations of chlorogenic acid reference solutions, prepared into sample solutions as per the method under "Preparation of solutions" and quantitatively determined according to the conditions under "Chromatographic conditions". The results showed that the average recovery was 98.2%, with a RSD=2.1%.
Sample determination: Lycium barbarum L. extract powder was weighed and prepared into sample solution as per the method under "Preparation of solutions". Each 10 μl of sample and reference solutions was injected for determination. Peak areas were recorded, and chlorogenic acid content in Lycium barbarum L. extract was calculated to be 34.91 mg/g.
Animal grouping and administration: The rats were randomly divided into blank control group, ocular hypertension model group and Lycium barbarum L. extract treatment group, n=10 in each group. All animals were fed and watered ad libitum. The rats in the treatment group were intragastrically administered 15 mg/100 g Lycium barbarum L. extract from 7 d after modeling once daily for 1 month. The control and model groups did not receive any medication.
Modeling method: Three episcleral veins in the eyes of rats were cauterized by Akira method [13].
IOP measurement: IOP was measured once 3 d before modeling and before administration (7 d after modeling) with Tono-pen XL tonometer, then measured once every 7 d for 1 month after administration.
mfERG test: In the treatment group, mfERG test was performed on both eyes one month after medication, while the other groups were tested at the same time. Rats were anesthetized by left lower intraperitoneal injection of 3% pentobarbital sodium solution at 1.5 ml/kg. Pupils for surgery were sufficiently dilated to 2~3 mm using tropicamide eye drops. Meanwhile, conjunctival surface was anesthetized with 1% tetracaine eye drops. The stimuli were 61 hexagons arranged in concentric circles, which were displayed on a 21 inch display at a 1: 4 contrast ratio. The stimulation was repeated for 47 S per period, and four periods were recorded for each test. Pass band was 50~300 Hz. The rats were placed on a plate about 20 cm in front of the display. The electrodes were all connected to acupuncture needles. R+ polar acupuncture needles were inserted into the inferior cornea margin of rats. R-polar acupuncture needles were inserted subcutaneously at the midpoint of a line connecting two supraorbital margins. The tips of needles were connected to the periosteum, while the ground electrode was placed subcutaneously at the tail root. The values were expressed in terms of amplitude density (nV/deg2) and latency (ms) of waves.
FCM detection of RGCs apoptosis rate: The rats in each group were excessively anesthetized within 24 h after the completion of mfERG test, and eyeballs were removed immediately. Under the microscope, cornea was cut at a site about 0.5~1 mm in front of the corneoscleral limbus to remove the lens and vitreous body. Retina was peeled off along the ora serrata and placed into 1 ml of PBS solution immediately. After cutting into pieces, the retina was digested with 1 ml of 0.25% trypsin, and placed in a 37°C CO2 incubator for 45 min, then the digestion was terminated with FBS, and the RGCs were washed with PBS and centrifuged at 2000 r/min for 3 min. The supernatant was discarded, and the remaining was washed twice, discarded of supernatant and fixed in 1 ml of 70% ice-cold ethanol (4°C), followed by FCM detection. Propidium Iodine (PI) staining was used: RGCs were stained with 1 ml of PI dye and kept in the dark at 4°C for 30 min. PI fluorescence was excited with an argon ion laser, where the laser wavelength was 488 nm, and the emission wavelength was over 630 nm. Red fluorescence was generated. The results were analyzed using ModFit software.
Determination of retinal NO content: After excessively anesthetizing the rats, eyeballs were removed immediately and placed on the ice (4°C). Under the microscope, cornea was cut at a site about 0.5~1 mm in front of the corneoscleral limbus to remove the lens and vitreous body. Afterwards, retina was peeled off along the ora serrata, weighed immediately, added with 1 ml of normal saline, fully homogenated and centrifuged at 3000 r/min for 15 min. The supernatant was collected and frozen, then determined by nitrate reductase assay.
Statistical methods
SPSS 11.0 was used to analyse the data. Before-after comparisons were made by paired t test, while intergroup comparisons were done by one-way ANOVA and LSD. The significance level was set at α=0.05.
Results
IOP
No significant difference was found in the IOP between various groups before modeling (P>0.05). IOP of treatment group before medication (7 d after modeling), model group and treatment group were all higher than that before modeling and the control group of corresponding period (P<0.01). There was no significant difference between the two groups (P>0.05), which were comparable. IOP of treatment group 1 month after medication and model group were lower than that 7 d after modeling (P<0.01) and was still higher than that before modeling and the control group of corresponding period (P<0.01). At that time, IOP of treatment group decreased compared to that before medication (P<0.01); was lower than the model group of corresponding period (P<0.01); and was close to that before modeling and control group of corresponding period (P>0.05). The results are shown in Table 1.
| Group | Number of eyes | Before modeling | 7 d after modeling (before medication) | 1 month after medication |
|---|---|---|---|---|
| Lycium barbarum L. extract treatment group | 20 | 12.54 ± 1.09 | 34.18 ± 4.15a,c | 14.57 ± 2.10b,d |
| Ocular hypertension model group | 20 | 12.34 ± 1.13 | 35.33 ± 4.65a,c | 26.23 ± 4.83a,b,c |
| Blank control group | 20 | 12.08 ± 1.41 | 13.09 ± 3.12 | 12.56 ± 2.47d |
| Note: Intragroup comparisons: Comparison with before modeling, aP<0.01; comparison with 7 d after modeling (before medication in the treatment group), bP<0.01 (paired t test). Intergroup comparisons: Comparison with the blank control group, cP<0.01; comparison with the ocular hypertension model group, dP<0.01 (one-way ANOVA, LSD). | ||||
Table 1. Contemporary comparison of IOP between treatment group and other groups before and after modeling and medication (͞x ± s, mmHg).
mfERG
The rats in the model group exhibited significantly longer total ring, two ring and four ring latencies of mfERG P1 wave than the control group (P<0.01), as well as significantly lower amplitude density (P<0.01). The above parameters were better for the treatment group than the model group, of which the total ring latency, amplitude density and two ring amplitude density differed significantly from the model group (P<0.05), while the total ring latency of P1 wave was similar to the control group (P>0.05). The results are shown in Table 2.
| Group | Number of eyes | Latency/ms | Total ring amplitude density/(nV • deg-2) | Two ring amplitude density/(nV•deg-2) |
Four ring amplitude density/(nV•deg-2) |
|---|---|---|---|---|---|
| Lycium barbarum L. extract treatment group | 20 | 45.30 ± 4.34a | 17.29 ± 10.34a,b | 32.19 ± 15.31a,b | 18.32 ± 8.12b |
| Ocular hypertension model group | 20 | 50.13 ± 8.21b | 12.53 ± 8.20b | 18.19 ± 14.13b | 13.42 ± 8.21b |
| Blank control group | 20 | 57.41 ± 24.09 | 44.87 ± 13.32 | ||
| Note: Comparison with the ocular hypertension model group, aP<0.05; comparison with the blank control group, bP<0.01 (one-way ANOVA, LSD) | |||||
Table 2. Comparison of mfERG P1 wave between various groups (͞x ± s).
RGC apoptosis rate
The apoptosis rate of RGCs in the model group was significantly higher than the control group (P<0.01). The apoptosis rate of treatment group was lower than the model group (P<0.05), while showing no significant difference from the control group (P>0.05). The results are shown in Table 3.
| Group | Number of eyes | RGC apoptosis rate |
|---|---|---|
| Lycium barbarum L. extract treatment group | 10 | 11.62 ± 7.38a |
| Ocular hypertension model group | 10 | 29.23 ± 13.09b |
| Blank control group | 10 | 6.75 ± 6.21 |
| Note: Comparison with the ocular hypertension model group, aP<0.05; comparison with the blank control group, bP<0.01 (one-way ANOVA, LSD) | ||
Table 3. Comparison of RGC apoptosis rate between groups (͞x ± s, %).
Retinal NO content
The NO content in the retina of the model group was significantly higher than the control group (P<0.01). Treatment group exhibited significantly lower NO content than the model group (P<0.05). The results are shown in Table 4.
| Group | Number of eyes | NO content/ (μmol/g) |
|---|---|---|
| Lycium barbarum L. extract treatment group | 10 | 18.61 ± 9.38a |
| Ocular hypertension model group | 10 | 31.03 ± 12.09b |
| Blank control group | 10 | 13.05 ± 6.21 |
| Note: Comparison with the ocular hypertension model group, aP<0.05; comparison with the blank control group, bP<0.01 (one-way ANOVA, LSD) | ||
Table 4. Comparison of retinal NO content between groups (͞x ± s).
Discussion
mfERG is a technique that can record many local electroretinograms simultaneously. [14] It can visually display the amplitude densities of corresponding sites on 3D topographic maps and can further analyse the amplitude density and latency of different quadrants and rings in the 2D diagrams. mfERG also allows analysis and comparison of a particular region and clear display of amplitude density decline and latency extension of dysfunctional sites. The results of this study showed that Lycium barbarum L. extract is conducive to the recovery of total ring latency and amplitude density of mfERG P1 wave, as well as amplitude density of two ring P1 wave. This suggested that the Lycium barbarum L. extract can partially improve the damage of persistent high IOP on the visual function of rats.
Regardless of the cause of glaucomatous optic nerve injury, its common feature is induction of RGC apoptosis [15], so the first step to the glaucomatous optic neuroprotection should be delayed apoptosis of RGCs. FCM not only can provide an objective evidence for cell apoptosis, but also allows quantitative analysis of apoptosis, which is thus a simple and feasible method to study the factors influencing apoptosis and its mechanism. FCM results in this paper revealed significantly higher apoptosis rate of RGCs in the model group than the control group (P<0.01), whereas lower RGC apoptosis rate in the treatment group than the model group (P<0.05). These suggested that the Lycium barbarum L. extract had a marked delaying effect on the apoptosis of RGCs in rats with persistent ocular hypertension. NO is the oxidation product of L-arginine in vivo produced by the catalytic action of Nitric Oxide Synthase (NOS), which is an important in-vivo mediator and plays an important role in regulating apoptosis of multiple cells. Recent studies have found that NO is an important regulator of retinal microcirculation, which can dilate blood vessels, increase blood supply and protect retinal nerve cells. Moreover, there are growing evidences [16] that NO is directly involved in the apoptosis of glaucomatous RGCs.
In this study, retinal NO content of rats in the model group was higher than the control group (P<0.01). Retinal NO content of the treatment group was lower than the model group (P<0.05), while showing no significant different from the control group (P>0.05). This result suggested that the ocular hypertension can lead to excessive NO production in the retina of rats to change the microenvironment of RGCs survival, thereby damaging RGCs. Lycium barbarum L. extract can lower the retinal NO content of ocular hypertension model rats or prevent its excessive generation, thereby maintaining or improving the RGC survival microenvironment; reducing NO's toxic effect; enhancing RGC's resistance to primary and secondary lesions; and preventing secondary damage of surrounding undamaged cells to prevent further neural degeneration. This should be one of the mechanisms of Lycium barbarum L. extract in intervening the RGC apoptosis.
Conclusion
Lycium barbarum L. extract has a protective effect on the optic nerve of rats with ocular hypertension, which is manifested primarily as improving the visual function of rats and preventing RGC apoptosis. Its mechanism may be associated with lowering of retinal NO content in rats and improvement of RGC survival microenvironment. The results of this paper provide the theoretical basis for the future clinical application of the herb for prevention and treatment of glaucomatous optic nerve damage.
References
- Pehlivan K, Coskun H, Saglam K, Bozat BG. Lycium barbarum L. (Goji berry) fruits improve anxiety, depression-like behaviors, and learning performance: the moderating role of sex. Turk J Biol 2016; 40: 762-771.
- Ahmed N, Wang M, Shu S. Effect of commercial Bacillus thuringiensis toxins on Tyrophagus putrescentiae (Schrank) fed on wolfberry (Lycium barbarum L.). Int J Acarol 2016; 42: 1-6.
- Zhang Q, Lv XL, Zhang M. Induction of apoptosis in human hepatoma SMMC-7721 cells by polysaccharides from Lycium barbarum L. Adv J Food Sci Technol 2016; 11: 784-791.
- Liu W, Liu YM, Zhu R, Yu JP, Lu WS, Pan C, Yao WB, Gao XD. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydrate Polymers 2016; 147: 114-124.
- Zhao J, Li H, Xi W, An W, Niu L. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem 2015; 173: 718-724.
- Mocan A, Vlase L, Vodnar DC, Gheldiu AM, Oprean R, Crian G. Antioxidant, antimicrobial effects and phenolic profile of Lycium barbarum L. flowers. Molecules 2015; 20: 15060-15071.
- Sylwia M, Michal Z. Chromatographic determination of phenolic acids and flavonoids in Lycium barbarum L. and evaluation of antioxidant activity. Food Anal Meth 2015; 8: 2665-2674.
- Mocan A, Vlase Laurian, Vodnar DC, Bischin C. Polyphenolic Content, Antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense mill. Leaves. Molecules 2014; 19: 10056-10073.
- Kosar M, Altintas A, Kirimer N, Baser KHC. Determination of the free radical scavenging activity of Lycium extracts. Chem Nat Comp 2003; 39: 531-535.
- Calzia D, Oneto M, Caicci F, Bianchini P, Ravera S, Bartolucci M, Diaspro A, Degan P, Manni L, Traverso CE, Panfoli I. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br J Pharmacol 2015; 172: 3890-3903.
- Fleming T, Martinez-Moreno CG, Mora J, Aizouki M, Luna M. Internalization and synaptogenic effect of GH in retinal ganglion cells (RGCs). Gen Comp Endocrinol 2016; 234: 151-160.
- Nickells RW. Retinal ganglion cell death in glaucoma: the how, the why, and the maybe. J Glaucoma 1996; 5: 345-356.
- Sawada A, Neufeld AH. Confirmation of the rat model of chronic, moderately elevated intraocular pressure. Exp Eye Res 1999; 69: 525-531.
- Kretschmann U, Seeliger M, Ruether K, Usui T, Zrenner E. Spatial cone activity distribution in diseases of the posterior pole determined by multifocal electroretinography. Vis Res 1998; 38: 3817-3828.
- Biswas SK, Zhao Y, Sandirasegarane L. Imatinib induces apoptosis by inhibiting PDGF- but not insulin-induced PI 3-kinase/Akt survival signaling in RGC-5 retinal ganglion cells. Mol Vis 2009; 15: 1599-1610.
- Jung MH, Seo YJ, Hye SL, Hwa KS, Dong HL, Sang HS, Chi DK, Sun SB. Regulation of retinal angiogenesis by endothelial nitric oxide synthase signaling pathway. Korean J Physiol Pharmacol 2016; 20: 533-538.