Short Article - Archives of Digestive Disorders (2017) Archives of Digestive Disorders (Special Issue 2-2017)
Roles of prostaglandin E and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract
Koji Takeuchi1,2*, Kikuko Amagase11Department of Pharmacology and Experimental Therapeutics, Kyoto Pharmaceutical University, Misasagi, Yamashina, Kyoto 607-8414, Japan
2General Incorporated Association, Kyoto Research Center for Gastrointestinal Diseases, Karasuma-Oike, Kyoto 604- 8106, Japan
- *Corresponding Author:
- Dr. Koji Takeuchi, PhD
General Incorporated Association
Kyoto Research Center for Gastrointestinal Diseases
Karasuma-Oike, Kyoto 604-8106
Japan
Tel: +81-077-545-5562
E-mail: takeuchi@mb.kyoto-phu.ac.jp
Accepted Date: May 05, 2017
Citation: Takeuchi K, Amagase K. Roles of prostaglandin E and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Arch Dig Disord. 2017;1(2):8-16.
Abstract
Endogenous prostaglandins (PG) play an important role in maintaining mucosal integrity and modulating the various functions of the gastrointestinal tract, and PGE2 is particularly effective in these actions. The receptors for PGE2 are pharmacologically classified into four specific G protein-coupled subtypes, EP1 to EP4, and the distribution of these receptors accounts for the multiple effects of PGE2 in the gastrointestinal tract. In recent years, studies using knockout mice genetically deficient of these receptors as well as their selective agonists and antagonists have gained much knowledge of the role of the PGE2 /EP receptors in gastrointestinal mucosal protection and healing. We herein review the roles of PGE2 in gastrointestinal mucosal protection and ulcer healing, focusing on the relationship with EP receptor subtypes.
Keywords
Mucosal protection, Prostaglandin, Ulcer, Prostanoids
Introduction
Prostaglandins (PGs), biosynthesized from arachidonic acid by cyclo-oxygenase (COX) and various PG synthesizing enzymes, are present throughout the gastrointestinal (GI) tract and bring about various actions, including the control of acid secretion, bicarbonate secretion, mucus production, and mucosal blood flow, and maintenance of mucosal integrity [1]. Indeed, PGs protects the GI mucosa against necrotizing agents, stress, and nonsteroidal anti-inflammatory drugs (NSAIDs). Robert et al. [2] first demonstrated that PGE2 protected the stomach from necrotizing agents, a phenomenon called "cytoprotection".
Currently, four specific G protein-coupled subtypes, EP1 to EP4, are known for PGE2 receptors, and the distribution of these receptors is considered to explain the multiple effects of PGE2 in various tissues including the GI tract [3,4]. All of these subtypes have been cloned, and knockout (KO) mice genetically deficient in each receptor have also been produced [5-7]. By using these KO mice the roles of specific PG receptors in various biological actions of PGs have been demonstrated [7-9]. We also performed a series of experiments to determine EP receptor subtypes that mediate the protective and healingpromoting effects of PGE2 in the GI tract, using various models in both rat and EP receptor KO mice [9-14]. Furthermore, highly selective EP agonists and antagonists have also been developed for some receptors and have greatly contributed to the elucidation of the pathophysiological roles of EP receptors in GI diseases (Table 1).
Table 1.Various subtype-specific EP receptor agonists and antagonists
| Prostanoids | EP Subtype Selectivity |
|---|---|
| 17-Phenyl PGE2 | EP1 agonist |
| Sulprostone | EP1/EP3 agonist |
| Butaprost | EP2 agonist |
| ONO-NT-012 | EP3 agonist |
| 11-Deoxy PGE2 | EP3/EP4 agonist |
| ONO-AE1-329 | EP4 agonist |
| ONO-AE1-734 | EP4 agonist |
| ONO-8711 | EP1 antagonist |
| ONO-AE-829 | EP1 antagonist |
| ONO-AE5-599 | EP3 antagonist |
| ONO-AE3-208 | EP4 antagonist |
| CJ42794 | EP4 antagonist |
We herein review, mainly based on our publications, the roles of PGE2 in gastrointestinal mucosal protection and ulcer healing, focusing on the relationship with EP receptor subtypes, and discuss possible functional mechanisms responsible for these effects of PGE2 in the stomach, duodenum, and small and large intestines.
Gastric Protection
Gastric lesions produced by necrotizing agents, NSAIDs and cold-restraint stress (CRS) are often used as experimental lesion models to investigate the protective action of PGE2 in the stomach [9-13,15]. We here introduced the protective effects of PGE2 on gastric lesions induced by NSAIDs and CRS.
Indomethacin-induced damage
NSAIDs damage the stomach of experimental animals and human as a major adverse event. There is no doubt that a deficiency of endogenous PG is a background factor in NSAIDinduced gastric lesions. Indeed, various NSAIDs such as indomethacin produced damage in the rat stomach at doses that significantly decreased the mucosal PGE2 concentration [12,16]. As expected, PGE2 exhibited a potent inhibitory effect on indomethacin-induced gastric damage. This effect of PGE2 was mimicked by 17-phenyl PGE2 and sulpropone, the prostanoids showing a potent affinity for EP1 receptors, but not by butaprost (EP2 anonist), ONO-NT-012 (EP3 agonist) or 11-deoxy PGE1 (EP3/EP4 agonist). In addition, the protective action of PGE2 against indomethacin was totally mitigated by prior administration of ONO-AE-829, a selective EP1 receptor antagonist [12]. On the other hand, indomethacin provoked gastric lesions in both wild-type and KO mice lacking EP1 or EP3 receptors, while the protective effect of PGE2 was observed in wild-type and EP3 receptor KO mice but not in mice deficient of EP1 receptors [13]. These results together strongly suggest that PGE2 prevents NSAID-induced gastric damage via the activation of EP1 receptors.
CRS-induced damage
CRS (10°C, 90 min) caused hemorrhagic lesions in wildtype mice, and the severity of these lesions was significantly worsened by the prior administration of indomethacin [15]. CRS-induced gastric ulcerogenic response was observed in EP1 or EP3 KO mice similar to wild type animals, but significantly worsened in mice lacking IP receptors. Iioprost, an analogue of PGI2, significantly reduced the severity of CRS-induced gastric lesions in wild type animals, in the absence or presence of indomethacin. This PGI2 analog shows an affinity not only for IP receptors but also for EP receptor as well [17]. However, iloprost did not affect the onset of CRS-induced gastric lesions in IP receptor KO mice, excluding the involvement of EP receptors in the prophylactic effect of this agent. In addition, the ulcerogenic response was not significantly influenced by the EP1 or EP3 antagonist, excluding the involvement of EP1 and EP3 receptors in mucosal protection during CRS. On the other hand, the CRS-induced gastric lesions was slightly aggravated by AE3-208 (EP4 antagonist), and the aggravation of these lesions by indomethacin was significantly mitigated by coadministration of AE1-329, the highly selective EP4 agonist [15]. Thus, endogenous PGE2 may also be partly involved in mucosal protection during CRS via the activation of EP4 receptors, in addition to PGI2/IP receptors. By the way, CRS-induced gastric ulcerogenic response was also significantly worsened by SC- 560 (COX-1 selective inhibitor), but not rofecoxib (COX-2 selective inhibitor) [15]. COX-2 expression was not detected in the stomach after CRS while COX-1 expression was observed under normal and CRS conditions. It is thus assumed that endogenous PGs derived from COX-1 plays an important role in protecting the gastric mucosa against CRS, and this effect is attributable mainly to PGI2 through IP receptors and party to PGE2 mediated by EP4 receptors.
Mechanism of Gastric Protection
Endogenous PGs are involved in the regulation of various gastric functions that contribute to gastric protection. According to previous studies [9,11,18-21], PGE2 increased mucus and HCO3- secretion through EP4 receptors, and inhibited acid secretion or motility through EP3 or EP1 receptors, respectively. The inhibitory effect of PGE2 on acid secretion was mediated in two ways by EP3 receptors, directly by inhibiting acid secretion at the parietal cells and indirectly by inhibiting histamine release at enterochromaffin-like (ECL) cells [22]. We also found that PGE2 stimulated acid secretion mediated by EP4 receptors through increase of histamine release from ECL cells [22]. Gastric mucosal blood flow was increased by EP2, EP3, and EP4 agonists, but not by EP1 agonists [9]. On the other hand, prostanoids showing an affinity for EP1 receptors inhibited gastric motility and protected the stomach against indomethacin-induced damage [12,13]. These effects were attenuated by ONO-AE-892, the EP1 antagonist, suggesting that the anti-motility effect of PGE2 parallels a reduction in the severity of NSAID-induced gastric lesions [12].
We reported that various compounds showed gastric protection at doses that inhibit gastric motility [23,24]. Suppression of gastric motility results in flattening mucosal folding and reducing mucosal vulnerability to irritants, and leads to prevention of the fold-related band-like lesions as seen after administration of NSAIDs such as indomethacin. A role of muscle elements in the pathogenic mechanism of NSAID-induced gastric damage has been demonstrated [16,25,26]. Mersereau and Hinchey [27] have shown for the first time that mucosal foldings due to stomach contraction are important in the occurrence of gastric lesions in response to NSAIDs. We reported that indomethacin at an ulcerogenic dose caused gastric hypermotility and resulted in microcirculatory disturbances due to abnormal mucosal compression of the stomach wall, preceding to the development of gastric lesions [25]. Although EP2, EP3 and EP4 agonists increased gastric mucosal blood flow, none of these agents had any effect on indomethacin-induced gastric lesions [9,14]. Therefore, it is unlikely that gastric protective effect of PGE2 against NSAID is functionally associated with an increase of gastric mucosal blood flow [9]. Because suppression of gastric motility may lead to amelioration of microvascular disturbances due to stomach contraction, it is possible that prostanoids can help to maintain mucosal blood flow indirectly through EP1 receptors during NSAID treatment.
The mechanism underlying the inhibition by PGE2 of gastric motility through EP1 receptor remains unknown. Milenov and Golenhofen [28] reported that PGE2 relaxed the circular muscle but contracted the longitudinal muscle of the canine stomach. Narumiya et al. [29,30] showed that strong signals for EP1 transcripts occurred in the smooth muscle cells within the muscularis mucosa throughout the GI tract. Because the activation of EP1 receptors increases phosphatidylinositol (PI) turnover [7], contraction of longitudinal smooth muscle by PGE2 is thought to be associated with an increase in cytosolic calcium. By contrast, contraction of circular smooth muscle leads to the emergence of mucosal foldings involved in the pathogenesis of NSAID-induced gastric damage [25-27]. At present, it remains unknown how PGE2 relaxes circular smooth muscle through the activation of EP1 receptors.
Neutrophils are also involved in the pathogenesis of NSAIDinduced gastric damage [31]. PGE2 inhibits neutrophil functions, including chemotaxis [12,32]. The inhibitory effect on neutrophil migration was similarly observed by butaprost and 11-deoxy PGE1, but not by 17-phenyl PGE2, sulprostone, or ONONT- 012 [12], suggesting the involvement of EP2/EP4 receptors in the anti-neutrophil action of PGE2. Since indomethacininduced gastric lesions were prevented by EP1 agonists but not EP2 or EP4 agonists [12], it is presumed that attenuation of neutrophil migration alone is not sufficient to suppress the overall expression of gastric lesions caused by indomethacin. Melange et al. [33] even reported using neutropenic rats that NSAID-induced gastric damage was neutrophil-independent. Because the increases in myeloperoxidase activity and lesion formation caused by indomethacin were both prevented when the enhanced gastric motility was inhibited by atropine [12,25,26], it is likely that neutrophil infiltration is secondary to the event associated with gastric hypermotility.
On the other hand, the exact mechanism how endogenous PGs contribute to mucosal protection during CRS remains unknown. Harada et al. [34] showed that CRS-induced gastric lesions were prevented by iloprost primarily through suppression of leukocyte accumulation. Because CRS-induced gastric damage is a neutrophil-dependent [1], it may also be possible that indomethacin aggravated these lesions by a decrease in PGI2 production and promoting the adherence of leukocytes to the vascular endothelium. PGE2 is also known to inhibit neutrophil migration via the activation of EP4 receptors [12]. This may support the protective effect of AE1-329, the EP4 agonist, on CRS-induced gastric lesions. On the other hand, Konturek and Robert [35] reported that PGI2 protected the gastric mucosa against ethanol by increasing mucosal blood flow. PGI2 and PGE2 increased mucosal blood flow in the rat stomach via the activation of IP and EP4 receptors, respectively [9,36]. Because iloprost did not increase gastric mucosal blood flow in IP receptor KO mice [37], dysregulation in gastric mucosal blood flow due to PG deficiency may be one of the mechanisms responsible for the increased ulcerogenic response to CRS in IP KO mice and in the presence of indomethacin. Furthermore, other studies showed an enhancement of gastric hypermotility as a pathogenic element in stress-induced gastric damage [38]. PGE2 inhibited gastric motor activity through the activation of EP1 but not EP4 receptors [9], excluding motility inhibition from the protective mechanism of PGE2 against CRS-induced gastric damage.
Duodenal HCO3- Secretion and Protection
Duodenal HCO3- secretion is an important process that helps to prevent acid injury. This is understandable when considering the fact that the duodenum secretes more HCO3- when the mucosa is acidified [39]. The regulatory mechanism of this process involves both humoral and neuronal factors as well as PGs [40], and among them endogenous PGs are particularly important in the local control of this secretion. Indeed, PGE2 stimulate duodenal HCO3- secretion in various species and protects the mucosal epithelium against acid-induced injury [41]. In addition, it has been shown that COX-1 is a key enzyme for regulating the acid-induced HCO3- secretion and maintaining the mucosal integrity against acid in the duodenum [42,43].
PGE2 stimulated duodenal HCO3- secretion in both Ca2+- and adenosine 3',5'-cyclic monophosphate (cAMP)-dependent manners [8,10]. This effect was mimicked by sulprostone, ONO-NT012, 11-deoxy PGE1, and ONO-AE1-329, but was not by butaprost or 17-phenyl PGE2 [10]. These results suggest that PGE2 stimulates duodenal HCO3- secretion via the activation of both EP3 and EP4 receptors and intracellularly mediated by Ca2+ and cAMP. Four splicing variants exist for the EP3 receptor, each coupled to different signal transduction pathways. The EP3A receptor is associated with the activation of Gi protein, the EP3B and EP3C receptor coupled to the activation of Gs protein, resulting in stimulation of adenylate cyclase (AC) activity, and the activation of the EP3D receptor leads to an increase in intracellular Ca2+ by stimulating PI turnover via Gq protein [7]. Thus, EP3B, EP3C, and EP3D receptors may be involved in the HCO3- stimulating action of PGE2 in the duodenum. As expected, the stimulatory effect of ONO-AE1-329 (EP4 agonist) was significantly enhanced by pretreatment with isobutylmethylxanthine, a phosphodiesterase inhibitor, but not verapamil, Ca2+ antagonist [44]. In general, the activation of two different signaling pathways results in a synergistic response in pharmacological action. Since costimulation by both EP3 and EP4 agonists produces a synergetic increase in duodenal HCO3- secretion, the dysfunction of either the EP3 or EP4 receptor system results in a substantial decrease in the HCO3- response in the duodenum. We also observed that HCO3- secretion in the duodenum of wild-type mice increased in response to luminal perfusion of PGE2 and forskolin as well as mucosal acidification [8]. Certainly, the latter effect was inhibited by prior administration of indomethacin, confirming the involvement of endogenous PGs in this action. The HCO3- response to acid was also observed in EP1 receptor KO mice, but disappeared in animals lacking EP3 receptors, yet the acidification increased mucosal PGE2 levels to a similar extent in all groups. As expected, the HCO3- stimulatory effect of PGE2 was profoundly decreased in EP3- but not EP1-receptor KO mice while forskolin similarly stimulated HCO3- secretion in both groups of mice. These results strongly support the idea that HCO3- secretion in the duodenum is mediated by EP3 and EP4 receptors.
As mentioned above, HCO3- secretion plays an important role in protection of the duodenal mucosa against acid injury [8,39,45]. Perfusion with 20 mM HCl in the proximal duodenum for 4 h only resulted in a few hemorrhagic lesions in wild-type mice. This response was not influenced by gene disruption of EP1 receptors, but significanlty worsened in mice lacking EP3 receptors [8]. The duodenal ulcerogenic response to acid was also markedly increased by indomethacin in wild-type mice. In EP3 receptor KO mice a progressive disruption of the mucosal defense system to acid is caused by a decrease in HCO3- secretion, leading to an increase of the mucosal susceptibility to acid injury. In addition, the acid-induced duodenal damage was exacerbated by pretreatment with AE5-599 (EP3 antagonist) and AE3-208 (EP4 antagonist) as well as indomethacin [46]. These results confirm the importance of endogenous PGs in maintaining the duodenal HCO3- secretion and mucosal integrity against luminal acid and further show that the presence of EP3 and EP4 receptors is essential for these actions of PGs.
Small Intestinal Protection
NSAIDs such as indomethacin are known to cause intestinal lesions in experimental animals and humans. Several factors have been postulated as pathogenic elements of these lesions, including PG deficiency, bile acids, bacterial flora, and nitric oxide (NO) [47,48], yet the precise mechanisms remain unknown. However, since these events caused by NSAIDs were effectively prevented by supplementation with exogenous PGE2 [13,48,49], it is certain that PG deficiency plays a critical role in the background of these lesions.
Indomethacin caused hemorrhagic lesions in the small intestine, mainly jejunum and ileum, accompanied by an increase in enterobacterial invasion. Pretreatment of the animals with dmPGE2, a stable PGE2 analog, dose-dependently and significantly prevented the development of these intestinal lesions in response to indomethacin [13,50]. This effect of dmPGE2 was mimicked by ONO-NT-012 (EP3 agonist) and ONO-AE1-329 (EP4 agonist), but other prostanoids such as 17-phenyl PGE2 (EP1 agonist) or butaprost (EP2 agonist) did not. These results suggest that the protective effect of PGE2 against NSAIDinduced enteropathy is mediated by the activation of EP3 and EP4 receptors. Lubiprostone, a bicyclic fatty acid derived from PGE1 [51], also prevented indomethacin-induced enteropathy, and this effect was abrogated by the co-administration of AE3- 208, an EP4 antagonist, confirming the involvement of EP4 receptors in the intestinal protection afforded by E type PGs [52]. In addition, we also showed using EP receptor KO mice that dmPGE2 provided less protection against indomethacininduced intestinal lesions in the animals lacking EP3 receptors, although the agent exhibited marked inhibition in both wildtype and EP1 receptor KO mice [13]. The fact that even in EP3 receptor KO mice dmPGE2 provided partial protection against these lesions, supports the involvement of another EP receptor subtype, EP4, in the intestinal protective action of PGE2.
Mechanism of intestinal protection
Several factors are implicated in the etiology of NSAIDinduced intestinal lesions. Among them, enterobacteria and nitric oxide (NO) play a key pathogenic role in these lesions; the release of bacterial products such as endotoxin contributes to the occurrence of intestinal damage through overproduction of NO by up-regulating the expression of inducible NO synthase (iNOS) in the mucosa [49]. Indeed, these lesions were prevented when NO production was suppressed by iNOS inhibitors or dexamethasone, an inhibitor of iNOS expression [48,49,53,54]. It has also been suggested that NO interacts with superoxide radicals to produce cytotoxic peroxynitrite, which has a detrimental effect on the intestinal mucosal integrity. The generation of intestinal lesions as well as bacterial translocation and the up-regulation of iNOS/NO production following indomethacin treatment were prevented by supplementation with dmPGE2, confirming a close relationship between intestinal protection and prevention of bacterial invasion as well as iNOS/ NO production [49,55].
Mucin plays an important role in native host defense against intestinal pathogens and irritants. We found that dmPGE2, ONO-NT-012, and ONO-AEI-329 increased mucus secretion in the small intestine, suggesting the involvement of the EP3/ EP4 receptor in the stimulatory effect of PGE2 [13]. Belly and Chadee [56] reported that PGE2 coupled to EP4 receptors stimulates cAMP-dependent mucin exocytosis in the rat colon. It is possible that PGE2, by stimulating the secretion of mucus and increasing the thickness of the mucus gel, prevents bacterial invasion in the mucosa, which is responsible for excessive NO production through up-regulation of iNOS expression [48,49,54,55]. In addition, the secretion of intestinal fluid may also prevent the process of bacterial invasion by washing away these microorganisms. This secretion was increased by dmPGE2, ONO-NT-012, and ONO-AE1-329, suggesting the stimulation of this process by EP3 and EP4 receptors [13]. Since this event is mainly associated with intestinal Clsecretion, several studies examined the effects of PGE2 on Cl- secretion in the GI tract [52,57]. We recently reported that lubiprostone, a PGE1 analogue, increased Cl- secretion in the isolated preparation of rat ileum mucosa, and this action was alleviated by the EP4 antagonist ONO-AE3-208, confirming the mediation of intestinal fluid secretion by EP4 receptors [52]. Because prostanoids exhibiting a preference for EP3 and EP4 receptors stimulated intestinal mucus/fluid secretion and prevented indomethacin-induced intestinal lesions [13,52], it is likely that these processes contribute to the intestinal protective action of PGE2 through inhibition of bacterial invasion in the mucosa.
On the other hand, NSAIDs at the ulcerogenic dose caused intestinal hypermotility [13,48,52,58]. Because the spasmodic nature of the intestinal motility disrupts the unstirred mucus layer over the epithelium, the enhanced intestinal motility may also be one of the pathogenic mechanisms for NSAID-induced small intestinal damage. The intestinal hypermotility caused by indomethacin was suppressed by dmPGE2 and other prostanoids exhibiting a preference for EP4 but not EP3 receptors [13,48,52,58]. Since EP4 receptors are coupled to Gs protein/ adenylate cyclase, the inhibitory effect of PGE2 on intestinal hypermotility is mediated by an increase of intracellular cAMP.
Thus, intestinal protection by PGE2 may be functionally associated with stimulation of mucus and fluid secretion and suppression of intestinal hypermotility; the former two being mediated by both EP3 and EP4 receptors while the latter mediated by EP4 receptors. These functional changes strengthen the barriers against intestinal pathogens and irritants followed by suppression of bacterial invasion as well as iNOS up-regulation in response to indomethacin, and thereby result in prevention of the development of small intestinal lesions.
Large Intestinal Protection
Ulcerative colitis is a chronic inflammatory disease of unknown cause affecting the rectum and colon [59]. Experimental colitis induced by dextran sodium sulfate (DSS) shows characteristics resembling the features of human ulcerative colitis, such as erosion and ulcers as well as inflammatory cell infiltration [60]. Studies have shown that DSS-induced colitis in mice or rats was prevented by ONO-AE1-329 and exacerbated by ONO-AE3-208 [61-64]. Kabashima et al. [63] reported using mice lacking various subtypes of EP receptors that only EP4-deficient mice developed severe colitis with DSS treatment. They also showed that the severity of colitis in wild-type mice was aggravated by the repeated administration of ONO-AE3-208 (EP4 antagonist) and ameliorated by that of ONO-AE1-734 (EP4 agonist). Furthermore, Nitta et al. [62] reported the up-regulation of EP4 receptor expression during DSS treatment. Thus, endogenous PGE2 is presumed to protect against DSS-induced colitis, mainly through the activation of EP4 receptors. This idea is supported by the findings that NSAIDs aggravated DSS-induced colitis by inhibiting PG production due to suppression of both COX-1 and COX-2 [64,65].
PGE2 is known to inhibit inflammatory cytokines and stimulate mucus secretion in the GI mucosa through the activation of EP4 receptors [52,63,66]. Kabashima et al. [63] reported that ONO-AE3-208 enhanced and ONO-AE1-734 suppressed Th1 cytokine production in lamina propria mononuclear cells derived from the colon. In addition, several studies suggest a pathogenic role for enterobacteria in experimental colitis and inflammatory bowel diseases [59,67]. In fact, the antibiotic metronidazole prevented the occurrence of DSS-induced colitis [68,69]. As the mucus layer is a barrier to bacterial infiltration, PGE2 may interfere with bacterial infiltration by strengthening the mucus barrier through stimulation of mucus secretion. Tanaka et al. [65] reported that DSS-induced colitis was aggravated by NSAIDs and ameliorated by PGE2, in association with the decrease and increase in the mucin protein expression, respectively. A causal relationship between the increased mucus secretion and the prevention of bacterial infiltration has been demonstrated in the rat small intestine using dmPGE2 or several mucosal protective agents [13,52,68,70,71]. Although further studies are required to elucidate the mechanism underlying PGE2-induced colon protection, it is presumed that EP4 receptors play a crucial role in maintaining intestinal homeostasis by keeping mucosal integrity and down-regulating immune response.
Healing-Promoting Action
Healing of gastric ulcer was delayed by the administration of indomethacin, a conventional NSAID, given repeatedly after the ulceration in both rats and mice. This effect was reproduced by the administration of rofecoxib, a COX-2 selective inhibitor, but not SC-560, a COX-1 selective inhibitor [72-74], suggesting the involvement of COX-2/PGE2 in the mechanism of ulcer healing. Indeed, the healing of gastric ulcers was delayed in COX-2 KO mice [74,75]. The mucosal PGE2 content was increased after ulcer formation, and this response was inhibited by indomethacin and rofecoxib but not SC-560. The delayed healing caused by indomethacin was significantly reversed by the co-administration of 11-deoxy PGE1, the EP3/EP4 agonist, but not with other prostanoids including EP1, EP2, and EP3 agonists. In addition, the healing of gastric ulcers in rats and mice was also delayed by the repeated administration of selective EP4 antagonists, such as ONO-AE3-208 and CJ42794 [74,76]. Expression of vascular endothelial-derived growth factor (VEGF) and angiogenesis were both up-regulated in the ulcerated mucosa, and these events were inhibited by indomethacin and rofecoxib as well as EP4 antagonists [74]. It was also observed that the VEGF expression in primary rat gastric fibroblasts was increased by PGE2 or ONO-AE1-329 (EP4 agonist), and these responses were attenuated by the simultaneous administration of CJ 42794 [76]. These results confirmed the involvement of COX-2/PGE2 in the healing of gastric ulcers and further suggested that the healing-promoting action of PGE2 is brought about by stimulation of angiogenesis via the up-regulation of VEGF expression mediated by the activation of EP4 receptors.
Essentially similar results were obtained in the healing of small intestinal lesions caused by NSAIDs [77-79]. Small intestinal lesions induced by indomethacin (10 mg/kg) healed within 7 days after ulceration, decreasing to approximately 1/5 of the initial size. The healing process of these lesions was impaired by the repeated administration of indomethacin (2 mg/kg) given once daily after ulcer formation. The healing-impairment effect of indomethacin was reproduced by ONO-AE3-208 (EP4 antagonist), and reversed by the co-administration of ONOAE1- 329 (EP4 agonist) with indomethacin. Mucosal VEGF expression was also up-regulated in the small intestine after ulceration, peaked at day 3 and then declined. Changes in VEGF expression were parallel to those in mucosal COX-2 expression and PGE2 content. A low dose indomethacin reduced both VEGF expression and angiogenesis in the mucosa during the healing process, and these effects were reversed by co-treatment with EP4 agonists. These results suggest that endogenous PGE2 promotes the healing of small intestinal lesions mediated by the activation of EP4 receptors, and this action is functionally associated with stimulation of angiogenesis via the up-regulation of VEGF expression.
Because the EP4 receptors are coupled to the Gs protein and increase the intracellular levels of cAMP [7], the stimulatory effect of PGE2 on VEGF expression is thought to be mediated intracellularly by cAMP. Sonoshita et al. [80] demonstrated that the up-regulated VEGF expression by PGE2 occurs in association with the increased formation of cAMP via the activation of EP4 receptors in intestinal polyposis. Miura et al. [81] reported that the expression of COX-2 and VEGF colocalized in fibroblast-like cells in the ulcer bed of human gastric tissue. These findings suggest that endogenous PGE2 stimulates both VEGF expression and angiogenesis in the ulcerated mucosa through the activation of EP4 receptors. Immunohistochemistry revealed the co-localization of COX-2 and VEGF in ulcerated rat stomach and small intestine, confirming a close relationship between these two substances during ulcer healing [76,77]. The precise mechanism by which PGE2 stimulates VEGF expression mediated by EP4 receptors remains currently unknown. Ding et al. [82] reported that PGE2 up-regulated VEGF expression in gastric cancer cells via the trans-activation of epidermal growth factor (EGF) receptors. Other studies showed that PGE2 induced MAP kinase activation with or without the involvement of EGF receptor trans-activation [83,84]. Furthermore, it has been reported that PGE2 induced VEGF expression via SP-1-binding sites on the VEGF promoter through EP2/EP4 receptors in cAMP-dependent and PKA-dependent manners [85]. Hatazawa et al. [74] showed that VEGF production in rat gastric fibroblasts in vitro was stimulated by forskolin as well as dibutyryl cAMP.
Conclusion
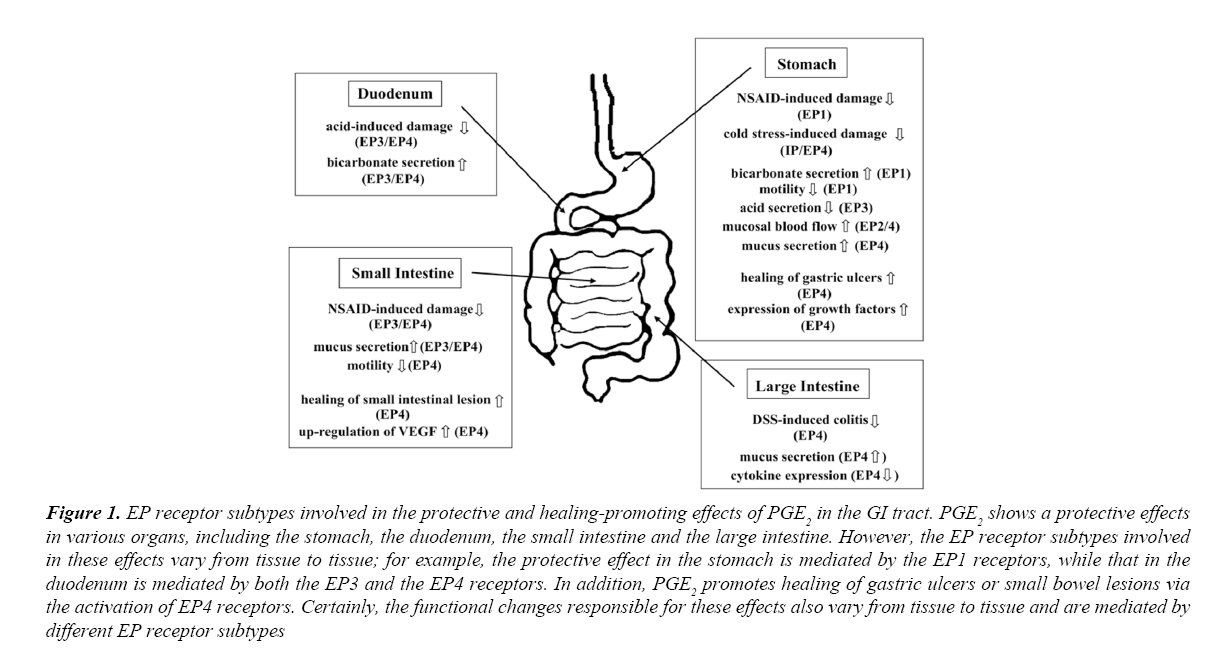
Endogenous PGs play a central role in the mucosal protection of the GI tract, in which PGE2 is the most important in their actions. As reviewed in this paper, PGE2 affords protection of the stomach against NSAIDs through the activation of EP1 receptors. Although CRS-induced gastric lesions were aggravated in IP but not EP1 KO mice, endogenous PGE2 may also be partly responsible for mucosal protection during CRS via the activation of EP4 receptors, in addition to that afforded by PGI2/IP receptors. On the other hand, PGE2 protects the duodenum against acid injury and the small intestine against NSAID-induced damage through the activation of both EP3 and EP4 receptors. The mechanisms responsible for these effects of PGE2 in the stomach, duodenum, or small intestine are suppression of gastric contraction (EP1), stimulation of duodenal HCO3- secretion (EP3/EP4) or suppression of bacterial invasion due to inhibition of intestinal motility (EP4) as well as stimulation of mucus secretion (EP3/EP4), respectively. PGE2 also preserves the large intestine (colon) from ulcers by activating the EP4 receptors, probably by maintaining mucosal integrity and down-regulating the immune response. In addition, PGE2 shows healing-promoting effects on gastric ulcers as well as small intestinal lesions through the upregulation of VEGF expression and stimulation of angiogenesis through the activation of EP4 receptors. It is worth noting that the EP receptor subtypes responsible for cytoprotection are different depending upon the tissues and that the functional alterations responsible for these actions differ depending on the tissues (Figure 1). It is therefore important to note that the EP receptor subtype involved in cell protection varies from tissue to tissue, and the functional changes involved in protective action vary from tissue to tissue. These findings contribute to further understanding of the mechanisms of "cytoprotection" and "healing-promoting action" of PGE2 in the GI tract and to the future development of new strategies for the treatment of GI disorders.
Figure 1: EP receptor subtypes involved in the protective and healing-promoting effects of PGE2 in the GI tract. PGE2 shows a protective effects in various organs, including the stomach, the duodenum, the small intestine and the large intestine. However, the EP receptor subtypes involved in these effects vary from tissue to tissue; for example, the protective effect in the stomach is mediated by the EP1 receptors, while that in the duodenum is mediated by both the EP3 and the EP4 receptors. In addition, PGE2 promotes healing of gastric ulcers or small bowel lesions via the activation of EP4 receptors. Certainly, the functional changes responsible for these effects also vary from tissue to tissue and are mediated by different EP receptor subtypes
Acknowledgments
We appreciate Professor Shu Narumiya, Kyoto University Faculty of Medicine, and Ono Pharmaceutical Co. Ltd. for generously supplying EP-receptor KO mice and various EP agonists and antagonists, respectively.
Conflict of Interest
The authors do not declare conflicts of interest.
References
- Miller TA. Protective effects of prostaglandins against gastric mucosal damage: Current knowledge and proposed mechanisms. Am J Physiol. 1983;245:601-23.
- Robert A, Nezamis JE, Lancaster C, et al. Cytoprotection by prostaglandins in rats: Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology.1979;77:433-43.
- Coleman RA, Kennedy I, Humphrey PPA, et al. Prostanoids and their receptors. In: Comprehensive medicinal chemistry, membranes & receptors. Edited by Emmett JC, Oxford, Pergamon Press. 1990;643-714.
- Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function.J Clin Invest. 2001;108:25-30.
- Sugimoto Y, Namba T, Honda A, et al. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem. 1992;267:6463-6.
- Watabe A, Sugimoto Y, Honda A, et al. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem. 1993;27:20175-8.
- Narumiya S, Sugimoto Y, Ushikubi F (1999) Prostanoid receptors: structure, properties and functions. Physiol Rev. 79:1193-6.
- Takeuchi K, Ukawa H, Kato S, et al. Impaired duodenal bicarbonate secretion and mucosal integrity in mice lacking prostaglandin E receptor subtype EP3. Gastroenterology. 1999;117:1128-35.
- Araki H, Yagi K, Suzuki K, et al. Roles of prostaglandin E receptor subtypes in cytoprotective action of prostaglandin E2 in rat stomachs. Alimental Pharmacol Ther. 2000;14:18-25.
- Takeuchi K, Yagi K, Kato S, et al. Roles of prostaglandin E-receptor subtypes in gastric and duodenal bicarbonate secretion in rats. Gastroenterology. 1997;113:1553-9.
- Takeuchi K, Araki H, Komoike Y, et al. Adaptive gastric cytoprotection is mediated by prostaglandin EP1 receptors: A study using rats and knockout mice. J Pharmacol Exp Ther. 2001;297:1160-5.
- Suzuki K, Araki H, Mizoguchi H, et al. E type prostaglandin inhibits indomethacin-induced gastric lesions in rats through EP1 receptors: Importance of antigastric motility action. Digestion. 2001;63:92-101.
- Kunikata T, Tanaka A, Miyazawa T, et al. 16,16-dimetyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig Dis Sci. 2002;47: 894-904.
- Takeuchi K. Gastric cytoprotection by prostaglandin E2: Relationship to EP receptor subtypes. J Physiol Pharmacol. 2014;65:3-14.
- Amagase K, Izumi N, Takahira Y, et al. Importance of cyclooxygenase-1/prostacyclin in modulating gastric mucosal integrity under stress conditions. J Gastroenterol Hepatol. 2014;29:S3-S10.
- Takeuchi K, Ueki S, Okabe S. Importance of gastric motility in the pathogenesis of indomethacin-induced gastric lesions in rats. Dig Dis Sci. 1986;31:1114-21.
- Narumiya S, Fitz Gerald GA. Genetic and pharmacological analysis of prostanoid receptor function.J Clin Invest. 2001;108:25-30.
- Yokotani K, Okuma Y, Osumi Y. Inhibition of vagally mediated gastric acid secretion by activation of central prostanoid EP3 receptors in urethane- anesthetized rats. Br J Pharmacol. 1996;117:653-6.
- Takahashi S, Takeuchi K, Okabe S. EP4 receptor mediation of prostaglandin E2-stimulated mucus secretion by rabbit gastric epithelial cells. Biochem Pharmacol. 1999;58:1997-2002.
- Ohno T, Katori M, Majima M, et al. Dilatation and constriction of rat gastric mucosal microvessels through prostaglandin EP2 and EP3 receptors. Aliment Pharmacol Ther. 1999;13:1243-50.
- Takeuchi K, Aihara E, Sasaki Y, et al. Involvement of cyclooxygenase-1, prostaglandin E2 and EP1 receptors in acid-induced HCO3- secretion in stomach. J Physiol Pharmacol. 2006;57:661-76.
- Kato S, Aihara E, Yoshii K, et al. Dual action of prostaglandinE2 on gastric acid secretion through different EP receptor subtypes in the rat. Am J Physiol. 2005;289:64-9.
- Takeuchi K, Matsumoto J, Ueshima K, et al. Gastric motility changes in the cytoprotective action of N-ethyl-maleimide and capsaicin in the rat stomach. In: Frontier of mucosal immunology, edited by Tsuchiya M, New York, Elsevier Science Publisher, 581-584.
- Takeuchi K, Niida H, Matsumoto J, et al. Gastric motility changes in capsaicin-induced cytoprotection in the rat stomach. Jpn J Pharmacol. 1991;55:147-55.
- Takeuchi K, Ueshima K, Hironaka Y, et al. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Digestion. 1991;49:175-84.
- Okada M, Niida H, Takeuchi K, et al. Role of prostaglandin deficiency in pathogenic Mechanism of gastric lesions induced by indomethacin in rats. Dig Dis Sci. 34:694-702.
- Mersereau WA, Hinchey EJ (1982) Role of gastric mucosal folds in formation of focal ulcers in the rat. Surgery. 1989;91:150-5.
- Milenov K, Golenhofen K. Contractile responses of longitudinal and circular smooth muscle of the canine stomach to prostaglandin E2 and F2a. Prostaglandins Leukotrines Med. 1982; 8:287-300.
- Ding M, Kinoshita Y, Kishi K, et al. Distribution of prostaglandin E receptors in the rat gastrointestinal tract. Prostaglandins. 1997; 53:199-216.
- Katori M, Majima M, et al. Dilatation and constriction of rat gastric mucosal microvessels through prostaglandin EP2 and EP3 receptors. Aliment Pharmacol Ther. 1999;13:1243-50.
- Wallace JL, Granger DN. Pathogenesis of NSAID gastropathy; are neutrophils the culpris? Trend Pharmacol Sci. 1992;3:129-31.
- Armstrong RA. Investigation of inhibitory effects of PGE2 and selective EP agonists on chemotaxis of human neutrophils. Br J Pharmacol. 1995;116:2903-8.
- Melange R, Gentry C, Toseland N, et al. Neutropenia does not prevent etodolac- or indomethacin-induced gastrointestinal damage in the rat. Dig Dis Sci. 1995;40:2694-703.
- Zimmerman BJ, Guillory DJ, Grisham MB, et al. Role of leukotriene B4 in granulocyte infiltration into the postischemic feline intestine. Gastroenterology. 1990;99:1358-63.
- Konturek SJ, Robert A. Cytoprotection of canine gastric mucosa by prostacyclin: possible mediation by increased mucosal blood flow. Digestion. 1982;25:155-63.
- Takeuchi K, Kato S, Takeeda M, et al. Facilitation by endogenous prostaglandins of capsaicin-induced gastric protection through EP2 and IP receptors. J Pharmacol Exp Ther. 2003;304:1055-62.
- Takeuchi K, Kato S, Ogawa Y, et al. Role of endogenous prostacyclin in gastric ulcerogenic and healing responses: A study using IP-receptor knockout mice. J Physiol Paris. 2001;95:75-80.
- Garrick T, Buack S, Bass P. Gastric motility is a major factor in cold restraint-induced lesion formation in: rats. Am J Physiol. 1986;150G191-9.
- Takeuchi K, Kita K, Hayashi S, et al. Regulatory mechanism of duodenal bicarbonate secretion: Roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther. 2011;130:59-70.
- Heylings JR, Garner A, Flemstrom G. Regulation of gastroduodenal HCO3- transport by luminal acid in the frog in vitro. Am J Physiol. 1984;246:235-46.
- Flemstrom G, Garner A. Gastroduodenal HCO3- transport: Characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982;242:G183-93.
- Hirata T, Ukawa H, Kato S, et al. Effects of selective cyclooxygenase-2 inhibitors on acid-induced alkaline secretory and mucosal ulcerogenic responses in the rat duodenum. Life Sci. 1997;61:1603-11.
- Takeuchi K, Kagawa S, Mimaki H, et al. COX and NOS isozymes involved in duodenal bicarbonate response induced by mucosal acidification in rats. Dig Dis Sci. 2002;47:2116-24.
- Aoi M, Aihara E, Nakashima M, et al. Participation of prostaglandin receptor EP4 subtype in duodenal bicarbonate secretion in rats. Am J Physiol. 2004;287:96-103.
- Takeuchi K, Furukawa O, Tanaka H, et al. A new model of duodenal ulcers induced in rats by indomethacin plus histamine. Gastroenterology. 198690:636-45.
- Aihara E, Nomura Y, Sasaki Y, et al. Involvement of prostaglandin E receptor EP3 subtype in duodenal bicarbonate secretion in rats. Life Sci.2007;80:2446-53.
- Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109-17.
- Takeuchi K, Satoh H. NSAID-Induced Small Intestinal Damage: Roles of various pathogenic factors. Digestion. 2015; 91: 218-32.
- Tanaka A, Kunikata T, Konaka A, et al. Dual action of nitric oxide in pathogenesis of indomethacin-induced small intestinal ulceration in rats. J Physiol Pharmacol. 1999;50:405-17.
- Kunikata T, Araki H, Takeeda M, et al. Prostaglandin E prevents indomethacin-induced gastric and intestinal damage through different EP receptor subtypes. J Physiol Paris. 2001;95:157-63.
- Schey R, Rao SS. Lubiprostone for the treatment of adults with constipation and irritable bowel syndrome. Dig Dis Sci. 2011;56:1619-25.
- Hayashi S, Kurata N, Yamaguchi A, et al. Lubiprostone prevents NSAID-induced small intestinal damage by suppressing the expression of inflammatory mediators via EP4 receptors. J Pharmacol Exp Ther. 2014;349:470-9.
- Boughton-Smith N, Evans SM, Laszlo F, et al. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br J Pharmacol. 1993;110:1189-95.
- Konaka A, Tanaka A, Kato S, et al. Nitric oxide, superoxide radicals and mast cells in pathogenesis of indomethacin-induced intestinal lesions in rats. J Physiol Pharmacol. 1999;50:25-38.
- Takeuchi K, Yokota A, Tanaka A, et al. Factors involved in up-regulation of inducible nitric oxide synthase in rat small intestine following administration of nonsteroidal anti-inflammatory drugs. Dig Dis Sci. 2006;51:1250-9.
- Belly A, Chadee K. Prostaglandin E2 stimulates rat and human colonic mucin exoxcytosis via the EP4 receptor. Gastroenterology. 1999;117:1352-62.
- Bunce KT, Spraggs CF. Prostanoids stimulation of anion secretion in guinea-pig gastric and ileum mucosa is mediated by different receptors. Br J Pharmacol. 1990;101:889-95.
- Takeuchi K, Miyazawa T, Tanaka A, et al. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion. 2002;66:30-41.
- Simmonds NJ, Rampton DS. Inflammatory bowel disease - a radical view. Gut. 1993;34:865-8.
- Elson CO, Sartor RB, Tennyson GS, et al. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-67.
- Morimoto K, Sugimoto Y, Katsuyama M, et al. Cellular localization of mRNAs for prostaglandin E receptor subtypes in mouse gastrointestinal tract. Am J Physiol. 1997;272:681-7.
- Nitta M, Hirata I, Toshina K, et al. Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: Colitis suppression by a selective agonist, ONO-AE1-329. Scand J Immunol. 2002;56:66-75.
- Hirata I, Murano M, Nitta M, et al. Estimation of mucosal inflammatory mediators in rat DSS-induced colitis: Possible role of PGE2 in protection against mucosal damage. Digestion. 2001;63:73-80.
- Okayama M, Hayashi S, Aoi Y, et al. Aggravation by selective COX-1 and COX-2 inhibitors of dextran sulfate sodium (DSS)-induced colon lesions in rats. Dig Dis Sci. 2007;52:2095-103.
- Kabashima K, Saji T, Murata T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883-93.
- Watanabe Y, Murata T, Amakawa M, et al. KAG-308, a newly-identified EP4 selective agonist, shows efficacy for treating ulcerative colitis and can bring about lower risk of colorectal carcinogenesis by oral administration. Eur J Pharmacol. 2015;754:179-89.
- Borody TJ, Warren EF, Leis S, et al. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42-7.
- Okayama M, Tsubouchi R, Nishio H, et al. Protective effect of intra-rectal administration of rebamipide on dextran sulfate sodium-induced rat colitis. Digestion. 2004;70:240-9.
- Rumi G, Tsubouchi R, Okayama M, et al. Protective effect of lactulose on dextran sulfate sodium-induced colonic inflammation in rats. Dig Dis Sci. 2004;49:1466-72.
- Kamei K, Kubo Y, Kato N, et al. Prophylactic effect of irsogladine maleate against indomethacin-induced small intestinal lesions in rats. Dig Dis Sci. 2008;53:2657-66.
- Amagase K, Kimura Y, Wada A, et al. Prophylactic effect of monosodium glutamate on NSAID-induced small intestinal damage in rats. Curr Pharm Design. 2014;20:2783-90.
- Ukawa H, Yamakuni H, Kato S, et al. Effects of cyclooxygenase-2 selective and nitric oxide-releasing nonsteroidal anti-inflammatory drugs on gastric ulcerogenic and healing response in experimental animals. Dis Dis Sci. 1998;43:2003-11.
- Halter F, Tarnawski AS, Schmassmann A, et al. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut. 2001;49:443-53.
- Hatazawa R, Tanaka A, Tanigami M, et al. Cyclooxygenase-2/prostaglandin E2 accelerates the healing of gastric ulcers via EP4 receptors. Am J Physiol. 2007;293:G788-97.
- Mizuno H, Akamatsu T, Kasuga M. Induction of cyclooxygenase-2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387-97.
- Takeuchi K, Tanaka A, Kato S, et al. Effect of CJ-42794, a selective antagonist of prostaglandin E receptor subtype 4, on ulcerogenic and healing responses in rat gastrointestinal mucosa. J Pharmacol Exp Ther. 2007;322:903-12.
- Hatazawa R, Ohno R, Tanigami M, et al. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-Induced small intestinal lesions in rats. J Pharmacol Exp Ther. 2006;318:691-9.
- Takeuchi K, Tanigami M, Amagase K, et al. Endogenous PGE2 accelerates healing of indomethacin-induced small intestinal lesions through up-regulation of VEGF expression via activation of EP4 receptors. J Gastroentrol Hepatol. 2010;25:67–74.
- Takeuchi K. Prophylactic effects of prostaglandin E2 on NSAID-induced enteropathy: Role of EP4 receptors in its protective and healing-promoting effects. Curr Opinion Pharmacol. 2014;19:38-45.
- Sonoshita M, Takaku K, Sasaki N, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc (Delta 716) knockout mice. Nature Med. 2001;7:1048-51.
- Miura S, Tatsuguchi A, Wada K, et al. Cyclooxygenase- 2-regulated vascular endothelial growth factor release in gastric fibroblasts. Am J Physiol. 2004;287:444-51.
- Ding YB, Shi RH, Tong JD, et al. PGE2 up-regulates vascular endothelial growth factor expression in MKAN28 gastric cancer cells via epidermal growth factor receptor signaling system. Exp Oncol. 2005;27:108-13.
- Krysan K, Reckamp KL, Dalwadi H, et al. Prostaglandin E2 activates mitogen-activated protein kinase/erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275-81.
- Mendez M, LaPointe M. CPGE2-induced hypertrophy of cardiac myocytes involves EP4 receptor-dependent activation of p42/44 MAPK and EGFR transactivation. Am J Physiol. 2005;288:2111-7.
- Bradbury D, Clarke D, Seedhouse C, et al. Vascular Endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcriptional factor binding sites. J Biol Chem. 2005;280:29993-30000.