- Biomedical Research (2014) Volume 25, Issue 2
Role of maternal serum ischemia modified albumin as a biochemical marker in preeclampsia.
Jyotirmayee Bahinipati1*, Prakash Chandra Mohapatra2 and Tapaswini Pradhan31Department of Biochemistry Kalinga Institute of Medical Sciences Bhubaneshwar Odisha India.
2Department of Biochemistry SCB Medical College and HospitalCuttack Odisha India.
3Department of Biochemistry Kalinga Institute of Medical Sciences Bhubaneshwar Odisha India.
- *Corresponding Author:
- Jyotirmayee Bahinipati
C/O Department of Biochemistry
Kalinga Institute of Medical Sciences
Bhubaneshwar, Odisha, India
Accepted date: September 07 2013
Abstract
Preeclampsia (PE) a pregnancy specific disorder is the most common cause of fetal and maternal death yet no specific prevention and treatment is available. Reliable biochemical markers for prediction and diagnosis of PE can have a better impact on maternal health and several of the markers have been suggested till now. Recently Ischemia Modified Albumin (IMA) has emerged as a marker in different diseases where ischemia is the origin or consequence behind disease pathology. Therefore this study was undertaken to evaluate the role of maternal serum IMA in PE and its correlation with the oxidative stress. 45 patients with PE were selected for the study and compared with 31 pregnant healthy controls. IMA IMA/Albumin and Malondialdehyde (MDA) with other routine biochemical markers were estimated in these patients. The results were then statistically analysed. IMA levels were found significantly raised in PE patients as compared to normal pregnant controls (p value <0.001). A significant correlation was also found between IMA levels and MDA levels in PE (r =+0.3 p value <0.05). Thus we conclude that IMA generated by hypoxia/ischemia driven oxidative stress is also raised in PE hence can be used as a biomarker in PE. Further studies are needed to establish the relationship between IMA and disease process and its association with severity of disease. Keywords: Preeclampsia Ischemia Modified
Keywords
Preeclampsia Ischemia Modified Albumin Malondialdehyde
Introduction
Normal placental development is essential to normal fetal development. Disorders which affect around a third of human pregnancies primarily include miscarriage and preeclampsia. Recent changes in human lifestyle such as delayed childbirth and hypercaloric diets may have increased the global incidence of placental-related disorders over the last decades. There is mounting evidence that oxidative stress or an imbalance in the oxidant/antioxidant activity in utero-placental tissues plays a pivotal role in the development of placental-related diseases. It is one of the most challenging complications of pregnancy affecting 2% of pregnancies is a major cause of maternal and perinatal morbidity and mortality [1].
During normal placental development there occurs trophoblastic invasion of maternal spiral arteries to allow increase of uterine blood supply necessary for maintaining pregnancy. Preeclampsia is associated with defective endovascular trophoblastic invasion and inadequate remodelling of uterine spiral arteries leading to hypoxic intrauterine environment and generation of oxidative free radicals [2]. Accumulation of biomarkers of oxidative stress accompanied by depletion of antioxidant reserves is considered as hallmark of preeclampsia [3-5].
As placental hypoxic conditions are found in preeclampsia and oxidative stress is implicated in its pathogenesis maternal serum Ischemia Modified Albumin(IMA) can be a potential biochemical marker of preeclampsia. Under physiological conditions the amino terminal of Human Serum Albumin (HSA) binds to transition metals. Ischemic reperfusion injury generates reactive oxygen species which modifies the N-terminal region of HAS, which reduces its capacity to bind to the transitional metals. This chemically changed albumin is called as ischemia modified albumin.
Keeping this in view this study was designed with an objective to estimate serum IMA and determine its benefit as a diagnostic and prognostic marker [6-8].
Materials and Methods
The present study included 45 preeclamptic women from OPD IPD and labour room of department of obstetrics and gynaecology as first diagnosed case of preeclampsia without any history of antihypertensive treatment and 31 healthy pregnant women. The cases and control group were age and gestational age matched.
Selection of preeclampsia cases were made with the criteria of pregnant women with blood pressure =140/90 mm of Hg with proteinuria > 0.3 gm/l or > 1+ measured by dipstick. Women with known medical conditions history of recurrent miscarriage hypertension or diabetes prior to pregnancy and gestational diabetes were excluded from the study. Blood was collected from preeclamptic cases at the time of admission before subjecting them to antihypertensive therapy. Ultrasonography was done to confirm the gestational age and exclude any obstetrical or gynaecological disorder.
Routine biochemical parameters like haemoglobinfasting blood sugar serum albumin serum urea creatinine uric acid were estimated in the venous blood of cases and control using FLEXOR –XL autoanalyser. Urinary protein was estimated by dipstick method. Serum IMA was done according to Bar Or et al 2000 [9]. Known amount of cobalt was added to serum sample & the unbound cobalt was measured by the intensity of coloured complex formed after reacting with dithiothreitol by spectrophotometer at 470nm. IMA values were expressed in U/ml. One IMA unit is defined as “µgm of free cobalt in the reaction mixture per ml of serum sample”. Serum IMA/Alb was then calculated. Serum MDA was estimated by Thiobarbituric Acid reactive substances by Satoh K [10]. Institutional ethical committee approval was obtained for the study and informed consent was taken from all women. All analyses were performed by using SPSS statistics package.
Results
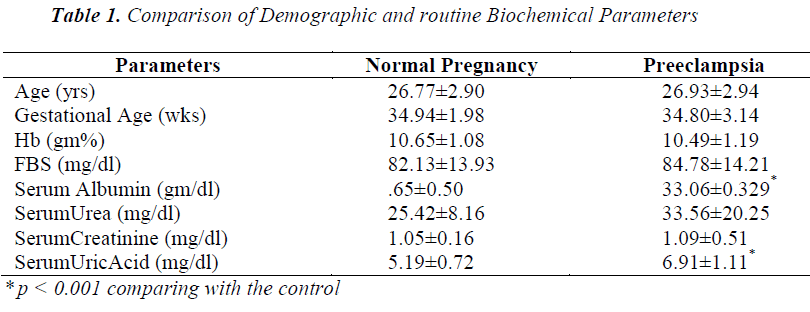
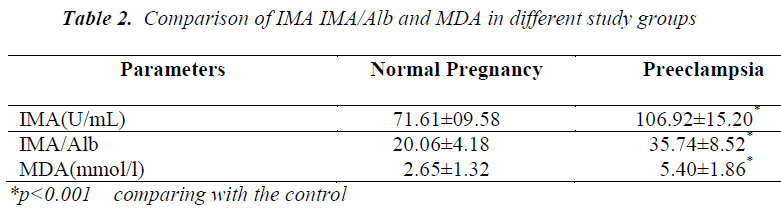
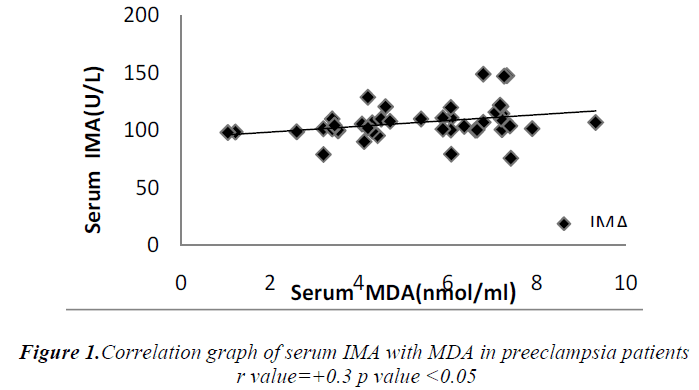
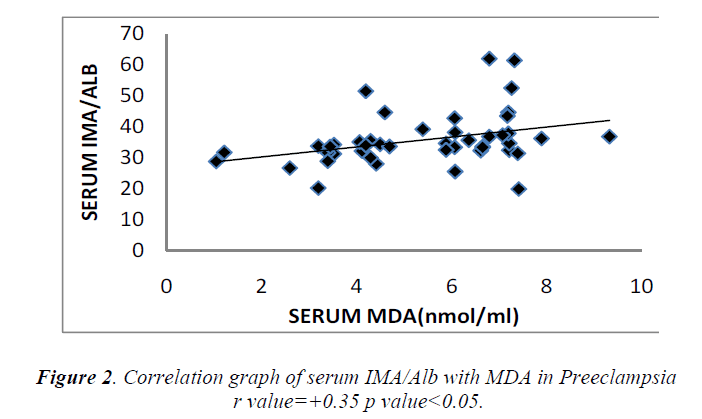
Cases and controls were age and gestational age matched. Table 1 shows the distribution of routine biochemical parameters in the study group. Results of serum albumin IMA IMA/Alb MDA are shown in the table 2. Mean serum IMA was elevated in preeclampsia (106.92±15.20 U/ml) as compared to normal pregnant women (71.61±9.58 U/ml p value<0.001). Significant positive correlation was seen between serum IMA and MDA (r=+0.30 p value<0.05) and between IMA/Alb and MDA (r=+0.35 p value<0.05) showing IMA originating from oxidative stress.
Discussion
Preeclampsia is associated with defective placentation leading to failure of conversion of small diameter high resistance vessels to large diameter low resistance vessels hence leading to ischemic reperfusion injury leading to oxidative stress and generation of free radicals [11,12]. Eclampsia is the end stage of the disease characterized by generalized seizures [13]. Pre-eclampsia and eclampsia complicate 2%–8% of pregnancies and overall 10%–15% of direct maternal deaths are associated with these conditions [14].
Biochemical markers not only allow detection of patients at risk but can also help in grouping patients into different categories according to their severity for timely intervention. Many different biophysical and biochemical markers have been investigated based upon pathophysiology of the disease but their reliability in predicting preeclampsia has been inconsistent.
Serum IMA has been observed to be significantly increased in diseases where oxidative stress is the consequence of the disease process. In the present study a significant rise in serum IMA was seen in preeclamptic women compared to normal pregnant women. There occurs hemodilution in pregnancy leading to decrease in plasma albumin concentration so IMA was normalized to albumin by calculating IMA/Albumin ratio. These observations were in accordance with the studies done by Gafsou et al [15] and Yusuf Ustun et al [16]. Placental hypoxia causing ischemic reperfusion injury results in the generation of free radicals which in turn causes alteration of NH 2 terminus of human serum albumin resulting in reduced binding of albumin to cobalt compared to normal pregnant control.
However in a limited study done by Van Rijn et al [17] serum IMA was found elevated in normal pregnant controls compared to the nonpregnant controls (p=0.015) but the IMA levels in preeclampsia were similar to those of normal pregnant controls (p=0.65). The discrepancy in these studies could possibly be explained by smaller number of patients and differences in severity of preeclampsia.
Significant increase in serum MDA was also observed in our study which was parallel to the observations of Ebru Dikensoy et al [18] YoneyamaY et al [19] and Mohd. Sahail et al [20]. These findings on maternal serum MDA provides further evidence that inappropriate or excessive lipid peroxidation may play an important role in pathophysiology of preeclampsia. There is significant positive correlation between the maternal serum IMA and MDA (Figure 1) as well as serum IMA/ALB and MDA in preeclampsia (Figure 2) suggesting that there occurs increase in serum IMA and IMA/ALB with increase in oxidative stress due to ischemia. This is in accordance with the work of Debasis Roy et al [21] who suggested that increased IMA levels may result from increased oxidative stress whether caused by ischemia reperfusion injury or other mechanisms linked to primary reduction in blood flow.
In conclusion IMA IMA normalized to albumin appears to be significantly increased in PE. This suggests that measurement of this oxidative biomarker may be useful in monitoring pregnancies with respect to the development of preeclampsia.
Acknowledgement
The contribution of participants who supported the study and the laboratory staff of SCB Medical College and Hospital Cuttack Odisha are duly acknowledged.
References
- Akolekar R, Syngelaki A, Sarq R. Prediction of early intermediate and late pre-eclampsia from maternal factors biophysical and biochemical markers at 11-13 weeks .Prenat Diagn 2011; 31: 66-74.
- Papageorghiou AT, Prefumo F, Leslie K, Gaze DC. Collinson PO Thilaganathan B. Defective endovascular trophoblast invasion in the first trimester is associated with increased maternal serum ischemia- modified albumin. Hum Reprod 2008; 23: 803-806.
- Roberts JM. Preeclampsia: What we know and what we do not know? Seminars Perinatology 2000; 24: 24-48.
- Uotila J Solakivi J JaakkolaT Tumila O Lehtimaki R. Antibodies Against copper oxidized and Malondialdehyde-Modified Low Density Lipoproteins in Preeclampsia Pregnancies. Br J Obstet Gynaecol 1998; 105: 1113-1117.
- Waish SW, Vaughan JE, Wang Y, Roberts LJ. Placental Isoprostane is significantly increased in preeclampsia. FASEB J 2000; 14: 1289-1296.
- Anwaruddin S, Januzzi JL, Baggish AL, Lewandrowski EL, Lewandrowski KB. Ischemia modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 2005; 123:140-145.
- Worster A, Devereaux PJ, Ansdell D, Guyatt GH, Opie J, Mookadam F, Hill SA. Capability of ischemia modified albumin to predict serious cardiac outcomes in the short term among patients with potential acute coronary syndrome. Can Med Assoc J 2005; 172: 1685-1690.
- Wu AH, Morris DL, Fletcher DR, Apple FS, Christenson RH, Painter PC. Analysis of the Albumin Cobalt Binding (ACB) test as an adjunct to cardiac troponin I for the early detection of acute myocardial infarction. Cardiovasc Toxicol 2001; 1: 147-151.
- Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt albumin binding and its potential as a marke for myocardial ischemia—a preliminary report. J Emerg Med 2000; 19: 311-315.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 1978; 90: 37-41
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006; 27: 939-958.
- Dekker GA Sibai BM. Etiology and pathogenesis of pre- eclampsia: current concepts. Am J Obstet Gynecol 1998; 179: 1359-1375.
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308:1592-1594.
- Duley L. The global impact of pre-eclampsia and eclampsia. Seminars in Perinatology 2009; 33:130-137.
- Gafsou B, Lefevre G, Hennache B, Debarge VH, Bouthers A. Maternal serum ischemia modified albumin; A biomarker to distinguish between normal pregnancy and preeclampsia. Hypertension in pregnancy 2010; 29: 101-111.
- Ustun Y, Ustun YE, Ozturk O, Alanbay I, Yaman H. Ischemia modified albumin as an oxidative stress marker in preeclampsia. Journal of maternal-fetal and neonatal medicine 2010; Early Online 1-4.
- Rijn V, Franx A, Sikkema JM, Herman JM, Bruinse H, Hieronymus Voorbij AM. Ischemia Modified Albumin in Normal Pregnancy and Preeclampsia: Hypertension in pregnancy 2008; 27: 159-167.
- Dikensoy E, Balat O, Pence S, Balat A. The changes of plasma malondialdehyde Nitric oxide and adrenomedullin levels in patients with preeclampsia. Hypertension Pregnancy2009; 28: 383-389.
- Yoneyama Y, Sawa R, Suzuki S, Koi D, Yoneyama K. Relationship between plasma malondi-aldehyde levels and adenosine deaminase activities in preeclampsia. Clin Chim Acta 2002; 322: 169 -173
- Suhail M, Suhail MF. Maternal and cord blood malondialdehyde and antioxidant vitamin levels in normal and preeclamptic women. BiochemiaMedica 2009; 19:182-191.
- Roy D Quiles J Gaze DC Collinsion P Kaski JC Baxter GF. Role of reactive oxygen species on the formation of novel diagnostic marker ischemia modified albumin. Heart 2006; 92: 113-114.