Short Communication - Journal of Pharmacology and Therapeutic Research (2017) Volume 1, Issue 1
Role of histone deacetylases 6 (HDAC6) in cancers.
Ahmad R. Safa*
Department of Pharmacology and Toxicology, Indiana University School of Medicine, Indianapolis, IN 46202, USA
- *Corresponding Author:
- Ahmad R. Safa
Department of Pharmacology and Toxicology
Indiana University School of Medicine
Indianapolis, IN 46202, USA
E-mail: asafa@iupui.edu
Accepted on December 20, 2017
Citation: Safa. Role of histone deacetylase 6 (HDAC6) in cancers. J Pharmacol Ther Res 2017;1(1):3-7.
DOI: 10.35841/pharmacology.1.1.3-7
Visit for more related articles at Journal of Pharmacology and Therapeutic ResearchIntroduction
MicroRNAs (miRNAs), acetylation of histones and non-histone proteins, and methylation of DNA serve as epigenetic factors. A balance between histone acetylases (HATs) and histone deacetylases (HDACs) exists in which these enzymes catalyze the addition or removal of acetyl groups on histones and other proteins and alter the stability and function of such proteins [1-4]. The mammalian HDAC family consists of 18 individual proteins based on their sequence homology, removing acetyl groups from lysine residues on histones and other proteins. The deacetylation can lead to modifications in various cellular functions including transcription, cell signaling, cell cycle kinetics, cell motility, cellular transport processes, and cell death [5,6]. HDACs consist of four different classes. Class I consists of HDAC1,-2,-3, and -8, which reside primarily in the nucleus, are found in various cells and tissues, and are predominantly responsible for targeting histones to repress gene transcription. Based on domain structure, Class II HDACs are subdivided into Class IIa (HDAC4,-5,-7, and -9) and Class IIb (HDAC6 and -10). These HDACs can move between the cytosol and the nucleus and HDAC6 is predominantly cytoplasmic [5]. Class III comprises the sirtuins, which act through a distinct NAD+-dependent mechanism and are not “classical” HDACs [1,5]. HDAC11 is the only member of Class IV as phylogenetic analysis revealed very low similarity to the HDACs in the other classes [5].
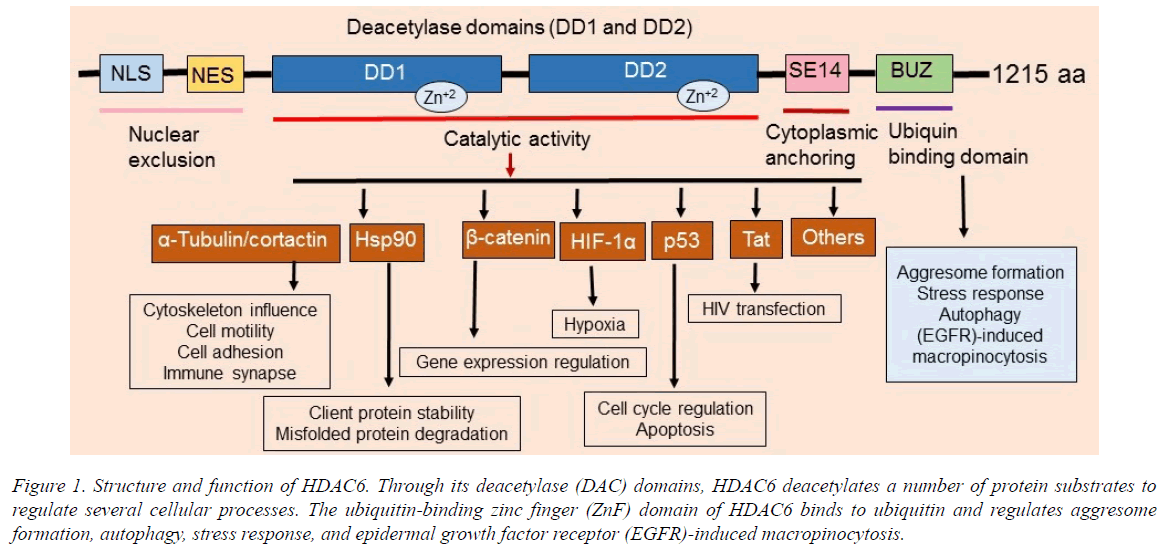
HDAC6 contains two tandem deacetylase domains and a C-terminal zinc finger, as well as an ubiquitin-binding domain (Figure 1). HDAC6 participates in tumorigenesis, cell motility, and metastasis. Therefore, HDAC6 has roles in a vast number of microtubule-dependent cytoplasmic processes. Significantly, since HDAC6 knockout mice are viable [4], it is an excellent target for cancer therapy. HDAC6 functions as an α-tubulin deacetylase [5] and deacetylates numerous substrates including cortactin, survivin, HSP90, Ku70, β-catenin, p53, HIF-1α, AKT, K-ras, Tat, 14-3-3ζ, Hsc70, HMGN2, Sam68, DNAJA1, MYH9, RIG-I, and others.
Figure 1. Structure and function of HDAC6. Through its deacetylase (DAC) domains, HDAC6 deacetylates a number of protein substrates to regulate several cellular processes. The ubiquitin-binding zinc finger (ZnF) domain of HDAC6 binds to ubiquitin and regulates aggresome formation, autophagy, stress response, and epidermal growth factor receptor (EGFR)-induced macropinocytosis.
Overexpression and increased function of HDAC6 is associated with the etiology of several cancers, systemic lupus erythematosus (SLE), inflammation, and central nervous system (CNS) disorders including Rett syndrome, Alzheimer's and Parkinson's diseases, and major depression disorder. It also deacetylates a number of critical cytoplasmic proteins involved in numerous signaling pathways, cellular functions, and biological processes (Figures 1 and 2). Therefore, there has been increasing interest in discovery of HDAC6 selective inhibitors as new therapeutic agents and chemical probes to understand the biological functions of HDAC6. Several studies [1,4-6] have implicated HDAC6 expression and function in regulating microtubules, growth factor-induced chemotaxis, misfolded protein stress response and tumor invasion, and it has been linked to cell transformation (Figure 1).
Lee et al., [7] reported that fibroblasts lacking HDAC6 display more resistance to both oncogenic Ras and ErbB2-dependent transformation, indicating a pivotal role for HDAC6 in oncogene-induced transformation. These authors also showed that HDAC6 is required for tumor cells to be resistant to anoikis (a programmed cell death that is initiated when cells detach from their peripheral matrix), therefore facilitating tumor cell invasion and metastasis [7].
HDAC6 regulates several important cellular processes and signaling pathways critical to physiological homeostasis in normal cells as well as in tumor initiation, promotion, proliferation, and metastasis [8] and is required for cancer stem cells (CSCs) maintenance [9]. Knockdown or inhibition of the function of HDAC6 promoted chemotherapy- as well as radiation-induced apoptosis, autophagy, senescence [8], and inhibits CSC stemness [9,10]. Deskin et al., [11] has showed that that HDAC6 mediates transforming growth factor β1 (TGF-β1)-induced epithelial to mesenchymal transition (EMT) in human lung cancer cells. Furthermore, inhibition of HDAC6 with tubacin or its silencing with siRNA attenuated TGF-β1- induced Notch-1 signaling [11]. This work suggests that HDAC6 may be an excellent therapeutic target for developing agents against tumor progression and metastasis.
HDAC6 is overexpressed in various cancer types [12-14] and its association with tumor prognosis and may be dependent on tumor type. For instance, Zhang et al., [12] reported that the expression levels of HDAC6 mRNA may have potential as an endocrine responsiveness marker as well as a prognostic indicator in estrogen receptor (ER)-dependent breast cancer possibly because HDAC6 is an estrogen-regulated gene [15].
However, further investigations are required to determine the relationship between HDAC6 expression and the response to endocrine therapy [12]. Interestingly, the immunohistochemical expression levels of HDAC6 and androgen receptor (AR) are correlated in ER-negative breast cancer and the expression of these proteins have negative prognostic value in predicting the overall survival of ERnegative breast cancer patients [13].
The HDAC6 expression levels in ovarian cancer cell lines and tissues are higher compared with benign lesions [16]. HDAC6 expression is also upregulated in primary oral squamous primary tumors [17]. HDAC6 is also overexpressed in primary acute myeloid leukemia (AML) blasts [18]. Temozolomide (TMZ)-resistant glioblastoma (GBM) cells express significantly more HDAC6 than their TMZ-sensitive counterparts and these TMZ-resistant cells showed more activation of the EGF receptor (EGFR). Interestingly, HDAC6 inhibitors decreased EGFR protein levels and impaired EGFR pathway activation [19]. Therefore, HDAC6 inhibition may represent a promising strategy for treating GBM. The upregulation of HDAC6 in diverse tumors and malignant cell lines suggests an important role of this protein in cancer.
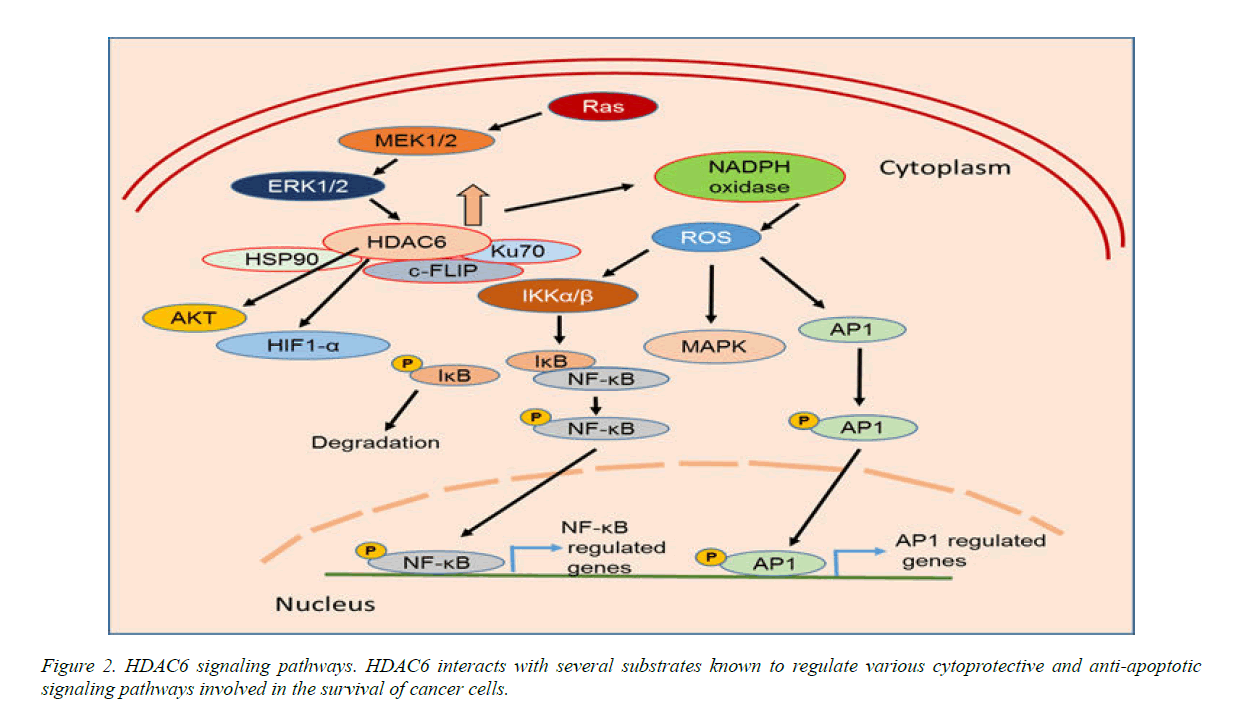
A considerable amount of data has shown that HDAC6 regulates p53 function, and directly or indirectly plays a role via HSP90 to stabilize hypoxia-inducing factor 1-α (HIF-1α), a transcriptional factor involved in tumor angiogenesis, Bcr-Abl, FLT-3, c-Raf and AKT (Fig. 2), and the breast cancer metastasis suppressor 1, BRMS1. HDAC6 also regulates the androgen receptor (AR) in cancer by modulating HSP90 acetylation. A recent study by Li et al., (13) evaluated HDAC6 and AR in 228 randomly selected cases of invasive breast cancer. High HDAC6 expression was significantly associated with high histologic grade (G3) (P<.001) and high AR expression levels (P<.01). These authors also showed that HDAC6 and AR have prognostic value in predicting the overall survival of ER-negative breast cancer patients.
Overexpression of HDAC6 is associated with an activated K-ras mutant in colon cancer patients. Moreover, expression of HDAC6 and c-myc are elevated in fibroblasts transformed with activated K-ras and mutant K-ras induces HDAC6 expression by a MAP kinase-dependent pathway [20].
Another interesting regulatory mechanism is EGF modulation of EGFR trafficking through intracellular sodium-mediated HDAC6 inactivation and tubulin acetylation [21]. Interestingly, HDAC6 participates in EGF-triggered β-catenin nuclear localization and activation of c-myc [22]. Mak et al., [23] demonstrated that HDAC6 physically associates with CD133 to negatively regulate CD133 trafficking down the endosomal-lysosomal pathway for degradation. Similarly, these authors showed that CD133, HDAC6, and β-catenin form a ternary complex [23]. Interestingly, HDAC6 is known to deacetylate β- catenin at lysine 49, which promotes β-catenin nuclear import [22].
HDAC6 overexpression significantly increased the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Increased expression of HDAC6 significantly induced reactive oxygen species (ROS) generation via upregulation of NADPH oxidase expression and activity (Figure 2). Furthermore, HDAC6 overexpression also increased activation of MAPK species including ERK, JNK, and p38. Therefore, HDAC6 regulates the ROS-MAPK-NF-κB/AP-1 pathways in Figure 2 [8-24].
Heat shock protein 90 (HSP90), acting as a chaperone protein known to safeguard proteins, is deacetylated by HDAC6. Recent data demonstrated the complexity and significance of the HDAC6-HSP90 interplay.Current data demonstrate that HDAC6 inhibitor-induced acetylation of HSP90 might control oncoproteins in malignant cells and serve as anti-cancer agents [25]. Therefore, combinations of HDAC6 and HSP90 inhibitors may synergistically trigger cell death and inhibit tumor growth.
HDAC6-selective and potent inhibitors including CAY10603, Tubacin, Tubastatin A, Rocilinostat (ACY-1215), and Nexturastat A provide promising new anti-cancer therapeutics [26], but their detailed mechanisms of action are not well elucidated. While there are several potent and selective inhibitors of HDAC6, the selective HDAC6 inhibitor ACY-1215 is being evaluated in clinical trials including multiple myeloma (MM) patients. ACY-1215 is cytotoxic for cultured and primary cells from patients with MM. ACY-1215 is known to shift cancer cells from antiapoptotic pathways to proapoptotic pathways.
Additionally, they trigger reactive oxygen species (ROS), inhibit DNA repair genes, and induce cell cycle arrest. Recent data using colorectal cancer cell lines revealed that the HDAC6-selective inhibitor A452 increased expression of wild-type p53 by destabilizing MDM2, but interestingly decreased mutant p53 by inducing MDM2 and inhibiting HSP90-mutant p53 complex formation [27]. Moreover, after treatment, HDAC6 expression levels inversely correlated with p53 acetylation at lysines 381/382 and were associated with p53 functional activation. A452 treatment increased levels of acetylated p53 at Lys381/382. A452 also disrupted the HDAC6-HSP90 chaperone complex via HSP90 acetylation and degradation. Bitler et al., [28,29] recently reported HDAC6 activity is essential in ARID1A-mutated ovarian cancers. ARID1A is a subunit of the SWI/SNF chromatin-remodelling complex which is the most frequently mutated epigenetic regulator among all human cancers. Inhibition of HDAC6 activity significantly improved the survival of mice bearing ARID1A-mutated tumors, but not wild-type ones. Therefore, HDAC6 inhibitors may serve as novel anticancer therapeutics regardless of the p53 mutation status of cancer.
Recent data demonstrated that HDAC6 is upregulated in human glioblastoma (GBM) stem cells (GSCs) compared to non-stem tumor cells [9] and its inhibition prevented radiosensitivity stemness and radioresistance of GSCs by downregulating glioma-associated oncogene homolog 1 (Gli1), Patched (Ptch1 and Ptch2) receptors and components of Sonic Hedgehog (SHH) signal expression and activity. HDAC6 inhibition decreased the stemness of GSCs and increased GSCs radiosensitivity through inactivating the SHH/Gli1 pathway. This provides a promising novel drug target to overcome GSCs stemness and radioresistance [9]. Therefore, HDAC6 is a promising therapeutic target for radiosensitization and reducing stemness in GSCs.
Cancer cells accumulate misfolded proteins at faster rate than normal cells [8] and they must dispose of these misfolded proteins through either the ubiquitin-proteasome system (UPS) or the aggresome-autophagy pathway [8,29]. Proteasome inhibitors are known to attenuate disposal of misfolded proteins by the UPS and their use in combination with HDAC6 inhibitors may enhance cytotoxicity by inhibiting both the UPS and the aggresome-autophagy pathway [30]. Indeed, HDAC6 inhibitors are being used in combination with proteasome inhibitors to treat lymphoma [31] and multiple myeloma [32].
A significant number of cancer patients develop severe side effects from certain anti-cancer drugs, and one of the most common neurological complications is painful peripheral neuropathy. Usually, chemotherapeutic agents that interfere with microtubules can cause these drug-induced peripheral neuropathies (CIPN). Recently, Van Helleputte et al., [33] reported that HDAC6-inhibitors offers neuroprotection without interfering with the anti-cancer efficacy of the anti-cancer agent vincristine. Therefore, HDAC6 inhibitors may offer a new and effective class of drugs that enhance chemotherapy-trigged cancer cell death as well as preventing the devastating chemotherapy-induced neuropathies.
In conclusion, HDAC6 is an unique isoenzyme, primarily expressed in the cytoplasm. Mice lacking HDAC6 are viable and develop normally, therefore it is an excellent target for developing inhibitors for cancer therapy. HDAC6 has specific substrates involved in protein trafficking, protein degradation, cell migration, and metastasis. HDAC6-selective inhibitors are an emerging class of therapeutics for cancer, neurodegenerative diseases, and immunological disorders.
References
- Li Y, Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Nat Cell Biol. 2017;19:962-973.
- Liang G, Weisenberger DJ. DNA methylation aberrancies as a guide for surveillance and treatment of human cancers. Epigenetics. 2017;12(6):416-432.
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381-395.
- Zhang Y, Kwon S, Yamaguchi T, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28(5): 1688-1701.
- Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol. Cell Biol. 2008;9(3):206-218.
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:(1)32-42.
- Lee YS, Lim KH, Guo X, et al. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res. 2008;68(18):7561-7569.
- Batchu SN, Brijmohan AS, Advani A. The therapeutic hope for HDAC6 inhibitors in malignancy and chronic disease. Clin Sci (Lond). 2016;130(12):987-1003.
- Yang W, Liu Y, Gao R, et al. HDAC6 inhibition induces glioma stem cells differentiation and enhances cellular radiation sensitivity through the SHH/Gli1 signaling pathway. Cancer Lett. 2017.
- Marampon F, Megiorni F, Camero S, et al. HDAC4 and HDAC6 sustain DNA double strand break repair and stemlike phenotype by promoting radioresistance in glioblastoma cells. Cancer Lett. 2017;397:1-11.
- Deskin B, Lasky J, Zhuang Y, et al. Requirement of HDAC6 for activation of Notch1 by TGF-β1. Sci Rep. 2016;6(1):31086.
- Zhang Z, Yamashita H, Toyama T, et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004;10(20):6962-6968.
- Li C, Cao L, Xu C, et al. The immunohistochemical expression and potential prognostic value of HDAC6, AR in invasive breast cancer. Hum Pathol. 2017.
- Sakuma T, Uzawa K, Onda T, et al. Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. Int J Oncol. 2006;29(1):117-124.
- Saji S, Kawakami M, Hayashi S, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24(28):4531-4539.
- Bitler BG, Wu S, Park PH, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol. 2017;19(8):962-973.
- Sakuma T, Uzawa K, Onda T, et al. Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. Int J Oncol. 2006;29:(1)117-124.
- Hackanson B, Rimmele L, Benkißer M, et al. HDAC6 as a target for antileukemic drugs in acute myeloid leukemia. Leuk Res. 2012;36:(8)1055-1062.
- Wang Z, Hu P, Tang F, et al. HDAC6 promotes cell proliferation and confers resistance to temozolomide in glioblastoma. Cancer Lett. 2016;379:(1)134-142.
- Wang Q, Tan R, Zhu X, et al. Oncogenic K-ras confers SAHA resistance by up-regulating HDAC6 and c-myc expression. Oncotarget. 2016;7(9):10064-10072.
- Lee SJ, Li Z, Litan A, et al. EGF-induced sodium influx regulates EGFR trafficking through HDAC6 and tubulin acetylation. BMC Cell Biol. 2015;16(1);16:24.
- Li Y, Zhang X, Polakiewicz RD, et al. HDAC6 is required for epidermal growth factor-induced beta-catenin nuclear localization. J Biol Chem. 2008;283(19):12686-12690.
- Mak AB, Nixon AM, Kittanakom S, et al. Regulation of CD133 by HDAC6 promotes β-catenin signaling to suppress cancer cell differentiation. Cell Rep. 2012;2(4): 951-963.
- Youn GS, Lee KW, Choi SY, et al. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-κB/AP-1 signaling pathways in macrophages. Free Radic Biol Med. 2016;97:14-23.
- Krämer OH, Mahboobi S, Sellmer A. Drugging the HDAC6-HSP90 interplay in malignant cells. Trends Pharmacol Sci. 2014;35(10)501-509.
- Wang XX, Wan RZ, Liu ZP. Recent advances in the discovery of potent and selective HDAC6 inhibitors. Eur J Med Chem. 2017.
- Ryu HW, Shin DH, Lee DH, et al. HDAC6 deacetylates p53 at lysines 381/382 and differentially coordinates p53- induced apoptosis. Cancer Lett. 2017;391:162-171.
- Bitler BG, Wu S, Park PH, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol. 2017;19(8):962-973.
- Rodriguez-Gonzalez A, Lin T, Ikeda AK, et al. Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res. 2008;68(8): 2557-2560.
- Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102(24):8567-8572.
- Amengual JE, Johannet P, Lombardo M, et al. Dual targeting of protein degradation pathways with the selective HDAC6 inhibitor ACY-1215 and bortezomib is synergistic in lymphoma. Clin Cancer Res. 2015;21(20):4663-4675.
- Vogl DT, Raje N, Jagannath S, et al. Ricolinostat, the first selective histone deacetylase 6 inhibitor, in combination with bortezomib and dexamethasone for relapsed or refractory multiple myeloma. Clin Cancer Res. 2017;23(13):3307-3315.
- Van Helleputte L, Kater M, Cook DP, et al. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol Dis. 2017;111:59-69.