- Biomedical Research (2014) Volume 25, Issue 1
Role of Habbatus sauda towards the histological features of nicotine treated male rats seminal vesicle and prostate gland.
S. Lina1*, N.H. Hashida2, and H. Eliza31Institute of Graduate Studies, 2Division of Biology, Center of Foundation Studies in Science, and
2Department of Anatomy, Faculty of Medicine, University of Malaya, 50603, Kuala Lumpur, Malaysia
- *Corresponding Author:
- S. Lina
Institute of Graduate Studies,
University of Malaya, 50603, Kuala Lumpur
Malayasia
Accepted date: September 08 2013
Citation: S. Lina, N.H. Hashida and H. Eliza. Role of Habbatus sauda towards the histological features of nicotine treated male rats seminal vesicle and prostate gland. Biomedical Research 2014; 25 (1): 11-18.
Abstract
Habbatus sauda (Nigella sativa) is a plant commonly used as herbal medicine for treatment of diseases while nicotine is an addictive chemical that is present in cigarettes. The study was conducted to observe the effects of Habbatus sauda on histological structures of nicotine treated male rats’ seminal vesicles (SV) and prostate glands (PG). Rats were divided into five groups: Habbatus sauda control (HSC), Habbatus sauda (HS), nicotine control (NC), nicotine (N) and nicotine – Habbatus sauda (NHS). The HSC and HS groups were force–fed with 0.1ml/100g of corn oil and 6μl/100g of Habbatus sauda oil, respectively. The NC and N groups were intramuscularly (i.m.) injected with 0.1ml/100g of saline and 5.0mg/100g of nicotine, respectively. The NHS group was treated with the same dosage of nicotine and Habbatus sauda as the N and HS groups. The treatment was conducted for 100 days. The PG and SV of animals in the N group showed reduction in the epithelial height of the mucosal linings compared to that of in the NC, HSC, HS and NHS groups. Moreover, there was also less acidophilic secretion materials found in the glands of the animals in the N group compared to the other 4 groups. However, the histology of the prostate glands and seminal vesicles in the NHS group was noted to be similar to that of in the control (NC and HSC) groups. Hence, this suggested that administration of Habbatus sauda could lead to an improvement in histology and function of both PG and SV in the nicotine treated rats.
Keywords
Nigella sativa, Habbatus sauda, nicotine, seminal vesicle, prostate gland, histology
Introduction
Infertility is considered as one of the public health issues recognised by World Health Organisation (WHO) where it affects approximately 10 – 15% of reproductive aged worldwide [1,2,and 3]. Many scientists prefer natural products to treat fertility problems as it is a natural primary source of fertility regulating agents [4]. Besides, studies showed those plant products have minimal or no negative implications as antifertility agents [4]. Interestingly, 25% - 30% of prescriptions in modern medicine also have active properties which derived from plants [5 and 6]. Habbatus sauda (Nigella sativa) from Ranunculaceae family is an annual herbaceous, dicotyledonous plant found growing annually in Eastern Europe, Middle East and Western Asia, as well as the bordering of Mediterranean Sea, Pakistan and India [7,8,9, and 10]. Habbatus sauda (HS) plant may grow with the minimum height of 20cm up to maximum height of 90cm [9]. The segment of HS`s leaves looks narrowly linear to thread – like. As for its flowers it differs in color with yellow, pink pale, blue or pale purple with each flower consists of 5-10 petals [9]. Fruits produced by N. sativa were located in a capsule where each capsule is composed with a few united follicles [9]. Inside the follicles is where numerous tiny black seed [7,9].In Arabic the tiny black seed of N. sativa is known as “Al-Habba Al-Sauda” and “Al-Habba Al-Barakah” in Arabic while in English it is called as black seed, black cumin or black caraway [7]. In some countries such as Asian and far Eastern countries, N. sativa is used as spice and food preservatives [10]. Dating back to 2 000 years ago, both seeds and oil of N. sativa are commonly used as natural health remedies in traditional medicine to treat numerous illnesses [10]. Researchers reported the active components that are present in N. sativa includes tymoquinone, 15 amino acids, proteins, carbohydrates, fixed oils, volatile oils, alkaloids, saponins, crude fiber, and minerals [7,10,11, and 12]. The reported pharmacological properties of N. sativa are anti – diabetic, anti – histamic, anti – oxidant, anti – inflammatory, anti – microbial, anti – tumor, and anti – fertility effects conducted in vivo and in vitro on laboratory animals and humans [7, 10, 13, and 14]. Studies showed that N. sativa, its oil or thymoquinone is quite safe when administered orally [7]. Study found that male rabbit that were given N. sativa in its meal for 6 weeks had better semen parameter [15]. However, studies on the effect of N. sativa on histological structure of seminal vesicle and prostate gland are still unclear. Moreover, study reported in Jordanian population Habbatus sauda was consumed as aphorodisiac and fertility promoting agent [38].
Male reproductive system is extremely sensitive and vulnerable towards both chemicals and drugs such as nicotine, ethanol, cocaine and cannabinoids [16]. Thus, cigarette smoking could result in negative effect on the reproductive system [17, 18, 19, and 20]. Cigarette smoke composed of more than 4000 chemical constituents where one of the compounds found in cigarette is nicotine [20, 21, and 22]. Nicotine is an alkaloid which derived from tobacco plant known as Nicotiana tabacum [23 and 24]. It acts on the central nervous system (CNS) – influencing drug [23 and 25]. Moreover, nicotine is also the main cause for repeated application among smokers due to its addictive nature [25]. One of the effects of nicotine at the cellular level is that it leads to oxidative stress which may have a detrimental result on the male reproductive system [18, 26, and 27]. Studies found that smoking result to detrimental effects on spermatogenesis [28].
The accessory glands present in male reproductive system are seminal vesicle and prostate gland. Both seminal vesicle and prostate gland are exocrine glands which have an important role in assisting the male fertility processes [29 and 30]. Seminal vesicle, measured about 10cm in length is a pair of simple tubular gland, saccular in shape and coiled in structure, positioned at the superior and posterior to the prostate, between the neck of urinary bladder of male mammals, found within the pelvis [31,32, and 33]. Seminal vesicle is a hormone – dependent gland which produces a viscous, yellow fructose – rich seminal fluid that represents approximately 60 – 70% of the volume of semen [30,31,32 and 33]. The secretion contains amino fructose (main constituent as it provide energy source of spermatozoa) amino acids, citrate, prostaglandin and proteins [30 and 31]. The role of seminal vesicle secretion was reported to be able to enhance the stability of sperm chromatin, together with to prevent the occurrence of immunity activity in the female reproductive tract [30]. Furthermore, it is suggested that any abnormal function of the gland may result either sexual dysfunction or infertility in males [30]. Prostate gland of sexually matured in mammals is the largest androgen – dependent male accessory sex glands [34,35,36 and 37]. Prostate gland consists of three lobes in rodents, namely the ventral, lateral and dorsal [36]. The names of the lobes were identified from the relation of prostate glanlobe is found on the inferior and posterior to the urinary bladder, below and behind the attachment of seminal vesicle and coagulating gland, while the lateral lobe lies immediately below seminal vesicle and coagulating gland with part of it overlapped with its ventral lobe and dorsal ld to the urethra [36]. The ventral lobe is located at the ventral aspect of urethra right below the urinary bladder; dorsal obe, dorsally [34 and 37]. Similar to seminal vesicle, rats prostate gland also have a role in secreting seminal fluid where secretion product from prostate gland is biochemically heterogeneous based on the origin of the prostate`s lobe where lobes of prostate known to have both morphological aspects as well as distinct hormonal responses [36 and 37]. Secretion of ventral lobe contains citrate, spermine, spermidine, aminopeptidases, and plasminogen activator while lateral lobe secretes major zinc- secreting portion [37]. As for the dorsal lobe of the gland, it secrets dorsal – protein I, dorsal – protein II, as well as is the major sites for fructose secretion [37].
The objectives of this study is to observe the improvement in the histological structure of nicotine – treated male rat`s reproductive system when administered with Habbatus sauda.
Materials and Methodology
Animals and Treatment
Healthy Sprague Dawley juvenile male rats (7 – 8 weeks old), weighing 150 – 250g obtained from the University of Malaya Medical Center (UMMC), University of Malaya. Prior to treatment, rats were allowed in the animal house for one week for familization with the animal house condition. Each rat was housed in a separate polypropylene cage with sawdust as bedding. It was maintained at room temperature with 12 hour of natural light and 12 hour of darkness. Chow food and tap water were given to rat ad libitum daily.
Experimental Design
Rats were randomly assigned into five groups; S: Nicotine control group received intramuscular (i.m.) injection of 0.1ml/100g body weight of saline, N: nicotine group received i.m. injection with 5.0mg/100g body weight of nicotine, CO: Habbatus sauda control group was force – fed with 0.1ml/100g body weight of corn oil, HS: Habbatus sauda group was force – fed with 6μl/100g body weight of Habbatus sauda oil and NHS: co – administration of both Habbatus sauda and nicotine with the same the dosage of 6μl/100g body weight of Habbatus sauda by force-feeding and 5.0mg/100g body weight of nicotine through i.m. injection. Habbatus sauda was obtained from Turkey while nicotine (L-Nicotine, 99+%, CAS RN: 54-11-5) which originate from alkaloid tobacco plants was purchased from Acros Organics. Each assigned groups (n = 6) were treated daily for 100 consecutive days and was sacrificed on the day 101. Animals were anesthetised via intraperitoneal (i.p.) injection with 3.5% of chloral hydrate (1.0ml/100g body weight). Rats were then sacrificed via intracardiac perfusion using 10% formalin solution into the vascular system. Rat seminal vesicle and prostate gland were harvested for histological study. Experiment procedures conducted were in accordance with the Guideline for Animal Experiment of the Medical Center Research Committee, University Malaya [ISB/20/04/2012/DSHA(R)].
Seminal Vesicle and Prostate Gland Histology
Harvested seminal vesicles were further fixed into 10% formalin solution in room temperature prior to histological processes. Tissues were dehydrated in graded alcohol solution and embedded in paraffin wax as blocks. Paraffin blocks with tissues were sectioned at 5μm thickness. Section tissues were stained with Haematoxylin and Eosin (H&E) and mounted with DPX (Dibutylphthalate Polystyrene Xylene) as mounting medium was obtained from Ajax Chemicals. Morphological structures of both seminal vesicle and prostate gland were observed under light microscope (Nikon Eclipse 80i) under 20x magnification.
Result
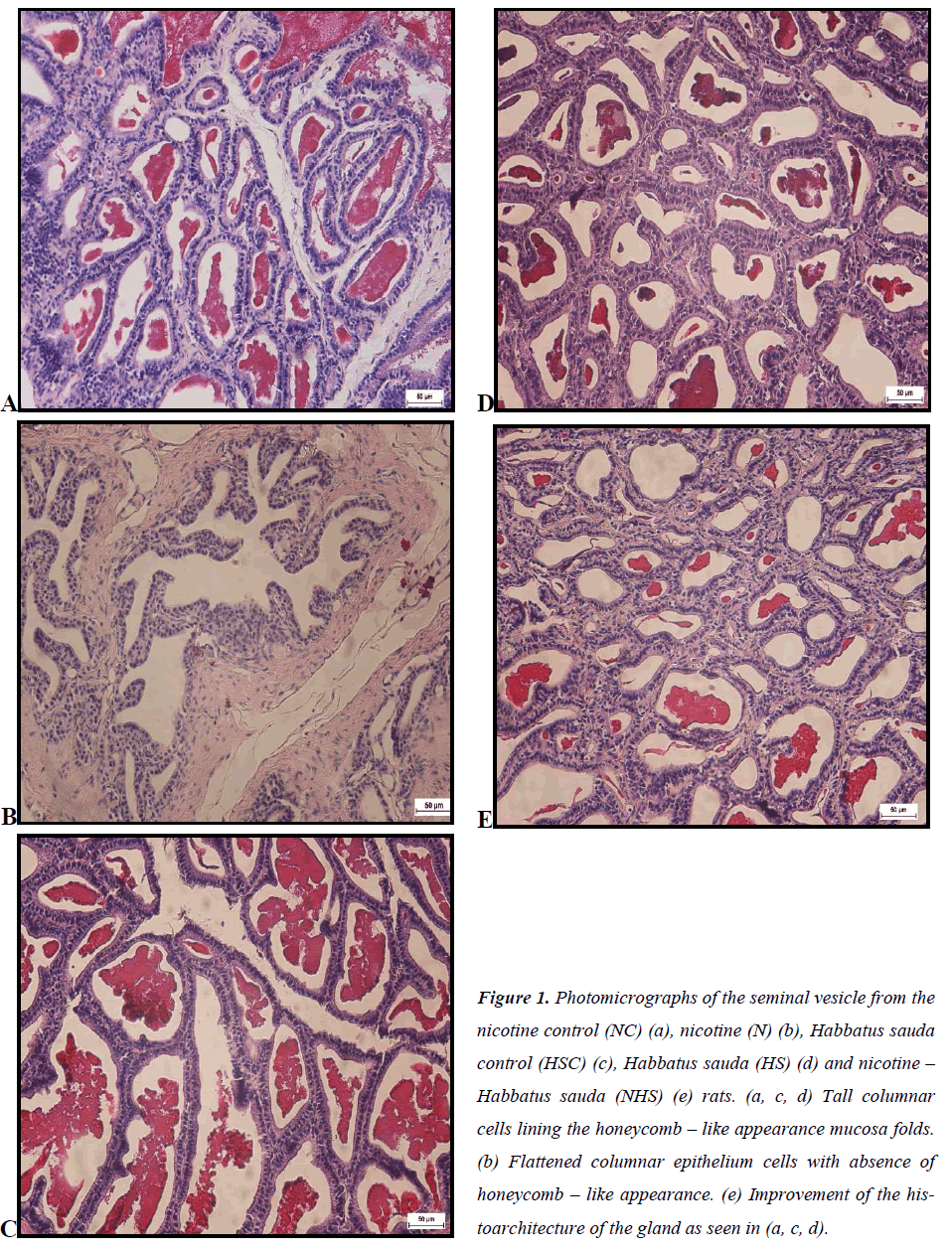
Histology of Seminal Vesicle Nicotine control (NC) group
Honeycomb – like appearance with anastomosing mucosal folds was visible in the NC group. Moreover, tall columnar cells were noticeable forming the thick epithelial wall. Acidophilic secretion also can be seen in Figure 1(a).
Nicotine (N) group
In seminal vesicle of the N group, it was noted that the honeycomb – like appearance was absence, as well as its acidophilic secretion in the lumen as in Figure 1(b). Asides from that, columnar cells of the N group also appeared to be flattened. The thickness of the epithelial wall however was undistinguishable compared to the control group.
Habbatus sauda control (HSC) group In Figure 1(c), the HSC group showed the presence of honeycomb – like appearance similar to the NC group. Furthermore, acidophilic secretion was also seen in its lumen, together with tall columnar cells which form epithelial lining.
Habbatus sauda group
Similar with its control group, seminal vesicle of the HS group also indicated the presence of honeycomb – like appearance accompanied with acidophilic secretion in the lumen seen in Figure 1(d). The columnar cells of the HS were noticeable to be tall in height.
Nicotine – Habbatus sauda group
The NHS group in Figure 1(e) indicated the presence of honeycomb – like appearance with tall columnar cells compared to the N group. In addition, acidophilic secretion in the lumen was also notable in this group compared to the N – treated group.
Figure 1: Photomicrographs of the seminal vesicle from the nicotine control (NC) (a), nicotine (N) (b), Habbatus sauda control (HSC) (c), Habbatus sauda (HS) (d) and nicotine – Habbatus sauda (NHS) (e) rats. (a, c, d) Tall columnar cells lining the honeycomb – like appearance mucosa folds. (b) Flattened columnar epithelium cells with absence of honeycomb – like appearance. (e) Improvement of the histoarchitecture of the gland as seen in (a, c, d).
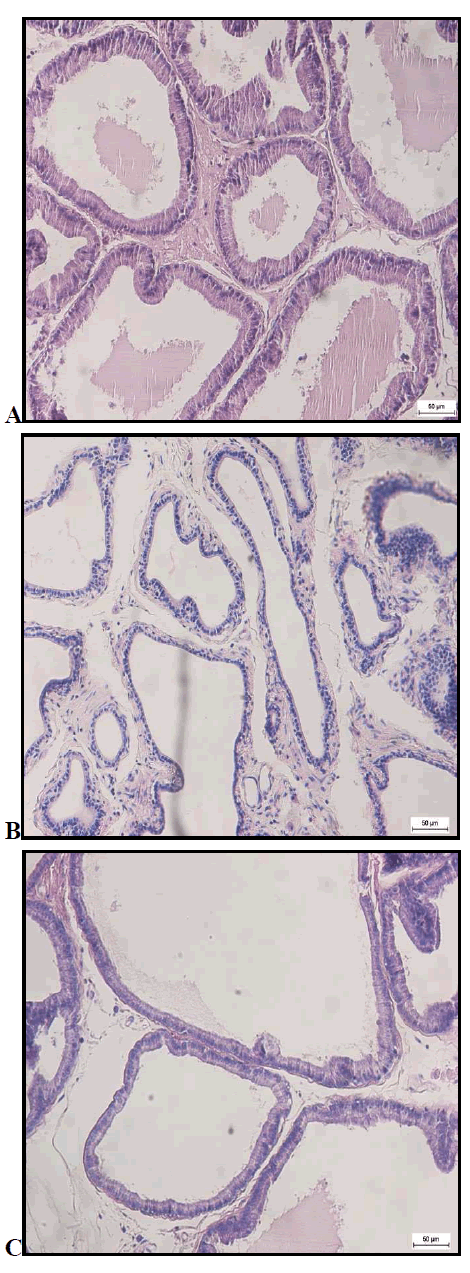
Histology of Prostate Gland
Nicotine control (NC) group
In Figure 2 (a), spaces between prostatic acini were relatively minimal, while the epithelial lining was formed by tall columnar cells. Acidophilic secretion was also noted in the lumen of prostate gland of the NC group.
Nicotine (N) group
Asides from not having mucosa glandular infoldings, it is visible that the spaces between the prostatic acini of prostate gland were seen to be increased in Figure 2 (b). In the same way, acidophilic secretion was also absence from its lumen. Likewise, the epithelium of the prostate gland in this treated group was flattened.
Habbatus sauda control (HSC) group
As shown in Figure 2 (c), mucosa glandular infoldings were within view. Besides, it was noted that the spaces between the prostatic acini were minimal, indicating that the prostatic acini was tightly packed with each other in the HSC group. As for the epithelium, simple tall columnar cells were noted with some acidophilic secretion available in the lumen.
Habbatus sauda group
In Figure 2 (d), HS group, the presence of mucosa glandular infoldings were much more compared to its control group. Besides, it is also noticeable that the gland was packed with prostatic acini. In addition, the tall columnar cells of its epithelial lining compliments with the presence of acidophilic secretion in the lumen.
Nicotine – Habbatus sauda (NHS) group
Glandular lining was also presence in the NHS group as shown in Figure 2 (e). Other than the existing of the mucosa glandular linings, the spaces between the prostatic acini were also quite tightly packed. Compared to the N group, the epithelium in the NHS was tall, columnar cells. However, similar to the N – treated, the NHS group also displayed the absence of acidophilic secretion in the lumen of the prostate gland.
Discussion
The present result on the adverse effects of nicotine towards prostate gland and seminal vesicle were similar as studies reported by previous researches [18,39, and 40]. This was in agreement with the review that stated an increase in the levels of reactive oxygen species (ROS) in the seminal fluids of smokers [18]. Favaro and Cagnon (2006) reported that long term consumption of nicotine would have led to detrimental effects on the male reproductive system including testis and prostate [36]. Besides,it was also stated that time of administration and nicotine dosage – dependent could result in changes of the body weight and male sexual organs (testis and accessory glands) [36 and 41].
In the histology of the prostate gland, it was clear that the tissue had less mucosa infoldings with flattened columnar cells of its epithelial lining in the N group. Correspondingly, studies by Favaro and Cagnon (2006) also reported that nicotine administration in rats have an unfavourable effects on the macroscopically features of prostate gland [36]. In the prostate gland of the N group, the absence of aal vesicle are both hormone – dependent organs. Hagras et al (2008) verified that changes in the male accessory sex organs could be due to reduction in output of the follicle stimulating hormone (FSH) and luteinising hormone (LH) from the pituitary gland which is vital tocidophilic secretion was also noted compared to the control group. This could be due to the fact that the accessory glands of male reproductive system; prostate gland and semin initiate spermatogenesis and steroidogenesis [39]. Previous studies showed that nicotine interfered with endocrine activities that were related to the reproductive function [42]. As nicotine is a central nervous system (CNS) influencing – drug, it inhibits the release of FSH and LH from pituitary [42]. Moreover, the epithelial height in the N group decreased compared to the other groups. Similar finding was reported by Londonkar et al. (2000) where there were significant decreases in the secretory activity and diameter of male accessory glands of nicotine treated animals [42].
As for the prostate gland of the NHS – treated group, it was noted that the height of epithelial walls increased compared to the N groups. Besides, the cells presence in the NHS group were tall columnar epithelial which was complimentary to that of in the HS group. This is probably due to the antioxidant pharmacological activities of the HS which would reduce the oxidative stress caused by nicotine. In agreement with the present study, Ghlissi et al. (2012) showed that the levels of antioxidant enzymes in male reproductive system of diabetic rats improved when administered with Habbatus sauda seed [43]. The prostate gland of the NHS group showed minimal spaces between prostatic acini similar as seen in the HS group. The reduction in the spaces between prostatic acini were also seen in a previous study which reported an improvement of acini in cancer – induced male rats when treated with Crateva nurvala plant [44].
The seminal vesicle in the N group showed a reduction in the height of the epithelial lining with flattened columnar cells. A similar finding was recorded by Londonkar et al. (2000) where nicotine was administered in male rats [42]. Lindane, which was claimed to trigger oxidative stress in various organs, also showed similar adverse effects of nicotine on the seminal vesicle [45]. The honeycomb – like appearance was visible in the NHS group but it was nowhere to be seen in the histological sections of the N group. Besides, in the NHS group, acidophilic secretion was also noted compared to the N group. A study reported that HS contained alkaloids and phenols as its active components which would stimulate the secretion of FSH and testosterone hormones [46]. Since the seminal vesicle is an androgen – dependent organ this would explain the presence of acidophilic secretion in the NHS group. It was reported that the secretion of seminal vesicle has an impact on the fertility and spermatogenesis. Thus HS could be beneficial in improving the sperm parameters in the nicotine – treated rats [47]. Moreover, the flattered height of seminal vesicle in the NHS group showed that HS improves the histological structure of seminal vesicle treated with nicotine. Previous study on seminal vesicle induced with cancer also stated an improvement in the height of the epithelium when the rats were administered with Crateva nurvala plant [44].
Conclusion
From the study, it showed that administration on nicotine without doubt would disrupt the morphological structures of the male accessory glands of reproductive system, subsequently leading to a decrease in male fertility. However, with the co-administration of HS, it is confirmed that the HS would be able to counteract and ameliorate the deleterious effects of nicotine towards the accessory glands of male reproductive system. Subsequently it would also improve the fertility of the reproductive system in mature adult male rats. However further study on the mode of action of HS on the accessory glands of male sexual system need to be investigated in details. In order to reduce the number of couples with infertility problems especially the men.
Acknowledgement
This work was supported under grant number: RG240- 12AFR and RG212/11AFR. Moreover, the authors would like to thank Department of Anatomy, Faculty of Medicine and Center of Foundation Studies, University Malaya, Kuala Lumpur for the facilities provided in the conduct of the research.
References
- Boivin J, Bunting L, Collins JA, and Nygren KG.International estimates of infertility prevalence and treatment- seeking: potential need and demand for infertility medical care. Human Reproduction 2007; 22 (6): 1506-1512.
- Deka PK, Sarma S. Psychological aspects of infertility.British Journal of Medical Practitioners 2010; 3(3): 32-34.
- Bushnik T, Cook JL, Yuzpe AA, Tough S, and Collins J. Estimating the prevalence of infertility in Canada. Human Reproduction 2012; 27(3): 738 –746.
- Bisht S, and Sharma N. Development and advancement in treatment of infertility: A review. International Journal of Drug Formulation and Research. 2010; 1 (II): 83 -100.
- Kumar D, Kumar A, and Prakash O. Potential antifertility agents from plants: a comprehensive review. Journal of Ethnopharmacology. 2012; 140: 1 – 32.
- Parandin R, Yousofvand N, and Ghorbani R. The enhancing effects of alcoholic extract of Nigella sativa seed on fertility potential, plasma gonadotropins and testosterone in male rats. Iran Journal Reproduction Medicine 2012; 10 (4): 355 – 362.
- Randhawa MA, and Alghamdi MS. Anticancer activity of Nigella sativa (Black seed) - a review. The American Journal of Chinese Medicine 2011; 39 (6): 1075-1091.
- Harzallah HJ., Grayaa R, Kharoubi W, Maaloul A, Hammami M, and Mahjoub T. Thymoquinone, the Nigella sativa bioactive compound, prevents circulartory oxidative stress caused by 1,2 – dimethylhydrazine in erythrocyte during colon post initiation carcinogenesis. Oxidative Medicine and Cellular Longevity 2012; 2012: 1-6.
- Al-Zuhairy RGM. The phytotherapeutic effect of traditional crude oil of Nigella sativa on male reproductive system of albino mice treated with low toxic dose of paracetamol. Iraqi Academic Scientific Journal. 2012; 9 (1): 229-237.
- Nehar S. and Kumari M. Protective effect of Nigella sativa seed oil on liver injury in rat. An International Quaterly Journal of Environmental Sciences 2012; 1: 409-412.
- Mishra RP. Effect of metal ions and Drugs on antibacterial activities of Nigella sativa (L.) seeds. ebmedCentral Ayurvedic Medicine 2011; 2(8): WMC00 2074.
- Al-Zahrani S, Mohany M, Kandeal S, and Badr G. Thymoquinone and vitamin E supplementation improve the reproductive characteristics of heat stressed male mice. Journal of Medicinal Plants Research.2012; 6 (3): 493-499.
- Sharma NK, Ahirwar D, Jhade D, and Gupta S. Medicinal and pharmacological potential of Nigella sativa: A review. Etnobotanical Review 2009; 13: 945 – 955.
- Mathur ML, Gaur J, Sharma R, and Haldiya KR. Antidiabetic properties of a spice plant Nigella sativa. Journal Endocrinology Metabolism 2011; 1(1): 1-8.
- El – Nattat WS, and El – Kady RI. Effect of different medicinal plant seeds residues on the nutritional and reproductive performance of adult male rabbits. International Journal of Agriculture and Biology 2007; 9 (3): 479-485.
- Sadeu JC, Hughes CL, Agarwal S, and Foster WG. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: reproductive health consequences and clinical implications. Critical Reviews in Toxicology 2010; 40 (7): 633-652.
- Dhawan K. and Sharma A. Prevention of chronic alcohol and nicotine – induced azospermia, sterility and decreased libido, by a novel tri – substituted benzoflavone moiety from Passiflora incarnata Linneaus in healthy male rats. Life Sciences 2002; 71: 3059- 3069.
- Tremellen K. Oxidative stress and male infertility – a clinical perspective. Human Reproduction Update 2008; 4 (3): 243-258.
- Calogero A. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reproductive Biomedicine Online 2009; 19 (4): 564- 571.
- Oyeyipo IP, Raji Y, Emikpe B.O, and Bolarinwa AF. Effects of nicotine on sperm characteristics and fertility profile in adult male rats: A possible role of cessation. Journal of Reproduction Infertility 2011; 12(3): 201-207.
- Fowles J. and Bates M. The chemical constituents in cigarettes and cigarette smoke: priorities for harm eduction. A Report to the New Zealand Ministry of Health. March 2000.
- Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos. Human Reproduction Update 2000; 6 (2): 122-131.
- Londonkar RL, Srinivasreddy P, Somanathreddy P, and Batil SB. Nicotine induced inhibition of the activities of accessory reproductive ducts in male rats. Journal of Ethnopharmacology 1998; 60: 215-221.
- Crooks PA and Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochemical Pharmacology 1997; 54: 743-753.
- Campain JA. Nicotine: Potentially a multifunctional carcinogen? Toxicological Sciences 2004; 79: 1-3.
- Yildiz D. Nicotine, its metabolism and an overview of its biological effects. Toxicon 2004; 43: 619-632.
- Sheweita S.A, Tilmisany AM, and Al – Sawaf H. Mechanisms of male infertility: role of antioxidants.Current Drug Metabolism 2005; 6: 1-7.
- Tziomalos K. and Charsoulis F. Endocrine effects of tobacco smoking. Clinical Endocrinology 2004; 61: 664-674.
- Wheater PR, Burkitt HG, Daniels VG. Male reproductive system. Functional Histology A Text and Colour Atlas. Hong Kong. English Language Book Society/Churchill Livingstone. Second Edition: Longman Group 1987; pp. 277-288.
- Noorafshan, A. and Karbalay – Doust, S. Curcumin protects the seminal vesicles from metronidazole –induced reduction of secretion in mice. Acta Medica 2012; 55: 32-36.
- Gartner, L.P. and Hiatt, J.L. Male reproductive system. Color Textbook of Histology. Third Edition.Saunders Elsevier. Philadelphia 2007; pp. 489-510.
- Akinsola AR, Oluwaseun H, Adewale H, Olusegun S, Adesina M. Effect of the methanolic Extract of Trichosanthes cucumerina seed (Snakegourd/Tomato) on hormone influenced seminal vesicle weight in adult Wistar rats. WebmedCentral Anatomy 2012; 3 (6): WMC003475.
- Lotti F, Corona G, Colpi GM., Filimberti E., Innocenti SD, Mancini M, Baldi E, Noci I, Forti G, and Maggi M. Seminal vesicles ultrasound features in a cohort of infertility patients. Human Reproduction.2012; 27 (4): 974-982.
- Hayashi N, Sugimura Y, Kawamura J, Donjacour AA, and Cunha GR. Morphological and functional heterogeneity in the rat prostatic gland. Biology of Reproduction 1991; 45: 308-321.
- Kumar VL and Majumder PK. Prostate gland: structure, functions and regulations. International Urology and Nephrology 1995; 27 (3): 231-243.
- Favaro WJ and Cagnon VHA. Morphometric and morphological features of the ventral prostate in rats submitted to chronic nicotine and alcohol treatment. Tissue and Cell 2006; 38: 311-323.
- Tlachi – Lopez JL, Lopez A, Hoffman K, Velazquez – Moctezuma J, Garcia - Lorenzana M., and Lucio RA. Rat dorsal prostate is necessary for vaginal adhesion of the seminal plug and sperm motility in the uterine horns. Bio Res 2011; 44: 259-267.
- Mohammad MA, Mohamad MMJ. and Dradka H. Effects of black seeds (Nigella sativa) on spermatogenesis and fertility of male albino rats. Research Journal of Medicine and Medical Sciences 2009; 4 (2): 386-390.
- Hagras GM, Ghobashy HEA., Kifafy MA., and Emara R. Histological and histochemical effects of nicotine on the adult albino rat prostate and the possible protective role of vitamin C. Menoufiya Medical Journal 2008; 21 (2): 307-326.
- Mahanem MN, Nor – Asmaniza AB, Phang HT, and Muhammad, H.R. Effects of nicotine and co –administration of nicotine and vitamin E on testis and sperm quality of adult rats. Malaysian Application Biology 2006; 35 (2): 47- 52.
- Carvalho CAF, Favaro WJ, Padovani CR and Cagnon VHA. Morphometric and ultrastructure features of the ventral prostate of rats (Rattus norvegicus) submitted to long – term nicotine treatment. Andrologia 2006; 38: 142-151.
- Londonkar RL, Sonar A, Patil S. and Batil SB. Nicotine delays puberty in male rat. Pharceutical Biology 2000; 38 (4): 291-297.
- Ghlissi Z, Hamden K, Saoudi, M, Sahnoun Z, Zeghal KM, Feki AE., and Hakim A. Effect of Nigella sativa seeds on reproductive system of male diabetic rats. African Journal of Pharmacy and Pharmacology 2012; 6 (20): 1444-1450.
- Kumar DG, Parvathi V, Meenakshi P, Rathi, MA, Gopalakrishnan VK. Anticancer activity of the ethanolic extract of Crateva nurvala bark against testosterone and MNU – induced prostate cancer in rats. Chinese Journal of Natural Medicines 2012; 10 (5): 334-338.
- Hfaiedh N, Murat J – C, and Elfeki A. Protective effects of garlic (Allium sativum) extract upon lindane – induced oxidative stress and related damages in testes and brain of male rats. Pesticide Biochemistry and Physiology 2011; 100 : 187-192.
- Al-Sa’aidi JAA Al – Khuzai ALD and Al – Zobaydi NFH. Effect of alcoholic extract of Nigella sativa on fertility in male rats. Iraqi Journal of Veterinar Sciences 2009; 23 (II): 123-128.
- Bahmanpour S, Vojdani Z, Panjehshahin mR, Hoballah H, and Kassas H. Effects of Carthamus tinctorius on semen gfd quality and gonadal hormone levels in partially sterile male rats. Korean Journal of Urology 2012; 53: 705-710.