Research Article - Biomedical Research (2017) Volume 28, Issue 16
Role of ErbB3 binding protein 1 (EBP1) as a predictor in hepatocellular carcinoma
Min-Lan Hu1#, Yan Qin2# and Xiao-Yan Wang3*
1Department of Ninth Section, No. 6 People’s Hospital, Qingdao, Shandong, PR China
2Department of Second Section, No. 6 People’s Hospital, Qingdao, Shandong, PR China
3Department of Hospital Infection-Control, No. 6 People’s Hospital, Qingdao, Shandong, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Xiao-Yan Wang
Department of Hospital Infection-Control
No. 6 People’s Hospital, PR China
Accepted date: July 26, 2017
Abstract
Background: The role of ErbB3 Binding Protein 1 (EBP1) in Hepatocellular Carcinoma (HCC) prognosis was inconsistent. Therefore, we performed this study to determine the effect of EBP1 in HCC prognosis.
Methods: 146 patients underwent resection of HCC were collected between 2010 and 2015. Overall Survival (OS) analysis was performed with Kaplan-Meier with log rank test. The prognostic value of EBP1 was evaluated by cox regression analysis.
Results: The expression of EBP1 was lower compared with adjacent normal tissues (p=0.01). EBP1 expression was significantly correlated with larger tumour size (p=0.03), metastasis (p=0.04), and advanced clinical stage (p=0.01). Univariate analysis of OS revealed that the relative level of EBP1 expression (p=0.002), tumor size (p=0.05), metastasis (p=0.04), and clinical stage (p=0.001) were prognostic indicators. Furthermore, multivariate analysis revealed that clinical stage (p=0.01) and EBP1 expression (p=0.03) were independent prognostic indicator for OS in HCC.
Conclusions: In conclusion, this study suggested that EBP1 expression was an independent prognostic indicator for OS in HCC.
Keywords
Hepatocellular carcinoma, ErbB3 binding protein 1, Overall survival
Introduction
Hepatocellular Carcinoma (HCC) is one of the most common cancers and ranks fifth among causes of cancer mortality worldwide [1]. Among the major risk factors for HCC, chronic infection with Hepatitis B Virus (HBV) is of particular interest for its coherent distribution with the HCC prevalence [2]. In Western countries and Japan, infection with HCV is the more common cause of HCC, while in Asia and developing countries, HBV is more common [3].
ErbB3 Binding Protein 1 (EBP1) is the human homologue of the mouse protein p38-2AG4, which regulates cell proliferation [4]. EBP1 also associates with mature ribosomes and suppresses the phosphorylation of the eukaryotic initiation factor 2 alpha under stress condition, and thus is possibly involved in the control of protein translation [5]. Ko et al. proposed that p42 Ebp1 functions as a potent tumor suppressor of non-small cell lung cancer through interruption of Akt signaling [6]. Awasthi et al. suggest that the ability of EBP1 to activate ErbB2 signaling pathways results in increased lapatinib sensitivity [7]. Ghosh et al. suggested that phosphorylation of EBP1 may be one mechanism of PAK1- induced hormone resistance and that PAK1 inhibitors may be useful in cells in which EBP1 is overexpressed [8]. Hu et al. indicated that EBP1 may be a valuable prognostic marker and promising therapeutic target of HCC [9]. However, the role of EBP1 in HCC prognosis was inconsistent. Therefore, we performed this study to determine the effect of EBP1 in HCC prognosis.
Materials and Methods
Patients and tissue samples
146 patients underwent resection of HCC were collected between 2010 and 2015. None of the patients received preoperative chemotherapy or radiotherapy. All tissue samples were immediately snap frozen in liquid nitrogen after surgery and stored at -80°C. All patients’ slides were reviewed to confirm the diagnosis and to classify the tumour according to the sixth edition of the Tumour Node Metastases (TNM) classification of the International Union against Cancer (UICC). The clinicopathologic features of the patients with HCC are shown in Table 1. Patients with evidence of other diseases were excluded from this study. Approval for the study was obtained from the Ethics Committee of Hospital.
| Characteristics | Low expression (n=71) | High expression (n=75) | P value |
|---|---|---|---|
| Age (y) | 0.33 | ||
| ≤ 55 | 36 | 32 | |
| >55 | 35 | 43 | |
| Gender | 0.27 | ||
| Male | 44 | 55 | |
| Female | 27 | 20 | |
| Tumor size (cm) | 0.03 | ||
| ≤ 5 | 28 | 43 | |
| >5 | 43 | 32 | |
| Tumor number | 0.11 | ||
| Solitary | 35 | 37 | |
| Multiple | 36 | 38 | |
| Metastasis | 0.04 | ||
| Yes | 54 | 29 | |
| No | 17 | 46 | |
| Clinical stage | 0.01 | ||
| I and II | 26 | 45 | |
| III | 45 | 30 |
Table 1: The relationship between EBP1 expression and clinicopathologic parameters.
Real-time quantitative RT-PCR for EBP1
EBP1 expression in tissues and cells was performed by realtime quantitative RT-PCR. Briefly, total RNAs were isolated from the tissues and cells by TRIzol (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol. Real-time (RT) and quantitative Polymerase Chain Reaction (qPCR) kits were used to evaluate the EBP1 expression from tissue samples. Primers for EBP1: 5’-TCAAAGCTGTAAGCTTATGTCGG-3’ and 5’-CATAAGAATTCCATACAAGGT-3’; β-actin: forward 5’-CCACTGGCATCGTGA TGGA-3’, reverse 5’- CGCTCGGTGAGGATCTTCAT-3’. Relative gene expression was calculated using the comparative Cycle Threshold (CT) (2-ΔΔCt) method, with β-actin served as an endogenous control for normalization.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). The significance of between-group differences was estimated using Student’s t-test and χ2 test, as appropriate. Overall Survival (OS) was calculated as the time interval from the date of surgery to either the day of the last follow-up or cancer-related death. OS rates were calculated using the Kaplan-Meier method, with the logrank test applied for comparison. Variables with a value of p<0.05 in univariate analysis were used in a subsequent multivariate analysis, based on the Cox proportional hazards model. Two-sided p-values were calculated, and P<0.05 indicated statistical significance.
Results
EBP1 expression and clinicopathologic factors of HCC
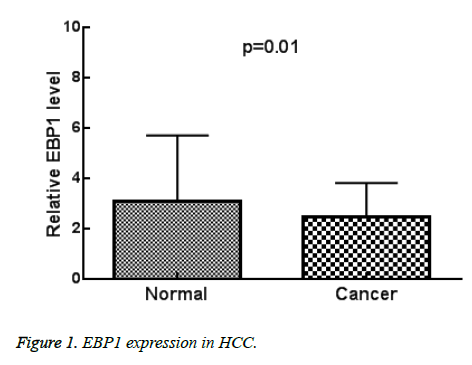
As shown in Figure 1, the expression of EBP1 was lower compared with adjacent normal tissues (p=0.01). As shown in Table 1, EBP1 expression was significantly correlated with larger tumour size (p=0.03), metastasis (p=0.04), and advanced clinical stage (p=0.01). No significant association was found between EBP1 expression and age (p=0.33), gender (p=0.27), tumor number (p=0.11).
EBP1 expression and prognosis of HCC
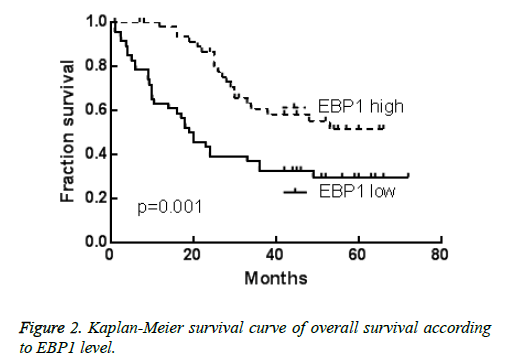
Kaplan-Meier method and log-rank test were used to investigate the prognostic value of EBP1 expression in HCC. As shown in Figure 2, HCC patients with low EBP1 expression were correlated with shorter OS (p=0.001) compared with those with high EBP1 expression. As shown in Table 2, univariate analysis of OS revealed that the relative level of EBP1 expression (p=0.002), tumor size (p=0.05), metastasis (p=0.04) and clinical stage (p=0.001) were prognostic indicators. Furthermore, multivariate analysis revealed that clinical stage (p=0.01) and EBP1 expression (p=0.03) were independent prognostic indicator for OS in HCC (Table 2).
| Prognostic factors | HR | 95% CI | P value | HR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Age (≤ 55/>55) | 1.15 | 0.63-1.92 | 0.62 | |||
| Gender (male/female) | 0.95 | 0.58-1.55 | 0.84 | |||
| Tumor size (≤ 5 cm/>5 cm) | 1.58 | 1.01-2.48 | 0.05 | |||
| Tumor number (solitary/multiple) | 1.26 | 0.96-1.64 | 0.09 | |||
| Metastasis (yes/no) | 2.67 | 1.07-6.67 | 0.04 | |||
| Clinical grade (I and II/III) | 2.66 | 1.48-4.80 | 0.001 | 2.11 | 1.16-3.94 | 0.01 |
| EBP1 (high/low) | 2.51 | 1.42-4.44 | 0.002 | 1.97 | 1.08-3.53 | 0.03 |
| HR: Hazard Ratio; CI: Confidence Interval. | ||||||
Table 2: Univariate and multivariate analysis for overall survival.
Discussion
In this study, we found that the expression of EBP1 was lower compared with adjacent normal tissues. EBP1 expression was significantly correlated with larger tumour size, metastasis, and advanced clinical stage. In addition, multivariate analysis revealed that clinical stage and EBP1 expression was independent prognostic indicator for OS in HCC.
Miao et al. suggested that EBP1 participates in the regulation of intestinal inflammation via mediating Akt signaling pathway [10]. Liu et al. suggested that Ebp1 suppressed thyroid cancer cell lines by upregulating RASRAL expression [11]. Gong et al. suggested that EBP1 is a novel prognostic indicator and potential therapeutic target of PDAC, shedding new insights into the important role of EBP1 in cancer development [12]. Nguyen et al. demonstrate an important role of Ebp1 in promoting cell proliferation in AML cells through the regulation of both rRNA synthesis and PCNA expression [13]. Wang et al. establish distinct physical and functional interactions between FBXW7 and EBP1 isoforms, which yield their mechanistically unique isoform-specific functions of EBP1 in cancer [14].
Several limitations were showed in the present study. Firstly, the sample size was relatively small. Secondly, the specific mechanisms for EBP1 regulating HCC were not investigated in the study.
In conclusion, this study suggested that EBP1 expression was an independent prognostic indicator for OS in HCC.
Disclosure of Conflict of Interest
The authors have declared that no competing interests exist.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74-108.
- Chu CJ, Lok AS. Clinical significance of hepatitis B virus genotypes. Hepatology 2002; 35: 1274-1276.
- Sarma MP, Asim M, Medhi S, Bharathi T, Diwan R. Viral genotypes and associated risk factors of hepatocellular carcinoma in India. Cancer Biol Med 2012; 9: 172-181.
- Radomski N, Jost E. Molecular cloning of a murine cDNA encoding a novel protein, p38-2G4, which varies with the cell cycle. Exp Cell Res 1995; 220: 434-445.
- Squatrito M, Mancino M, Sala L, Draetta GF. Ebp1 is a dsRNA-binding protein associated with ribosomes that modulates eIF2alpha phosphorylation. Biochem Biophys Res Commun 2006; 344: 859-868.
- Ko HR, Nguyen TL, Kim CK, Park Y, Lee KH. P42 Ebp1 functions as a tumor suppressor in non-small cell lung cancer. BMB Rep 2015; 48: 159-165.
- Awasthi S, Ezelle H, Hassel BA, Hamburger AW. The ErbB3-binding protein EBP1 modulates lapatinib sensitivity in prostate cancer cells. Mol Cell Biochem 2015; 405: 177-186.
- Ghosh A, Awasthi S, Peterson JR, Hamburger AW. Regulation of tamoxifen sensitivity by a PAK1-EBP1 signalling pathway in breast cancer. Br J Cancer 2013; 108: 557-563.
- Hu B, Xiong Y, Ni R, Wei L, Jiang D, Wang G, Wu D, Xu T, Zhao F, Zhu M, Wan C. The downregulation of ErbB3 binding protein 1 (EBP1) is associated with poor prognosis and enhanced cell proliferation in hepatocellular carcinoma. Mol Cell Biochem 2014; 396: 175-185.
- Miao X, Tang Q, Miao X, Wu Y, Qian J, Zhao W, Wang L, Li L, Zhang D. ErbB3 binding protein 1 (EBP1) participates in the regulation of intestinal inflammation via mediating Akt signaling pathway. Mol Immunol 2015; 67: 540-551.
- Liu H, Li Z, Li L, Peng H, Zhang Z. EBP1 suppresses growth, migration, and invasion of thyroid cancer cells through upregulating RASAL expression. Tumour Biol 2015; 36: 8325-8331.
- Gong C, Zhang Y, Chen Y, Zhang H. High expression of ErbB3 binding protein 1 (EBP1) predicts poor prognosis of pancreatic ductal adenocarcinoma (PDAC). Tumour Biol 2015; 36: 9189-9199.
- Nguyen le XT, Zhu L, Lee Y, Ta L, Mitchell BS. Expression and role of the ErbB3-binding protein 1 in acute myelogenous leukemic cells. Clin Cancer Res 2016; 22: 3320-3327.
- Wang Y, Zhang P, Wang Y, Zhan P, Liu C, Mao JH, Wei G. Distinct interactions of EBP1 isoforms with FBXW7 elicits different functions in cancer. Cancer Res 2017; 77: 1983-1996.