Research Paper - Archives of General Internal Medicine (2018) Volume 2, Issue 1
Role of BMI and Venous Resistance in Modulation of Cardiac Filling.
Gaston K Kapuku1*, Siva M Krothapalli1, Jim H Corley1, Umer Saleem1, Mahendra K Mandawat1,2, James D Halbert1, Harry Davis1 and Vincent JB Robinson1
1Medical College of Georgia, Augusta University, Augusta, USA
2Charlie Norwood Veteran Affairs Medical Center, Augusta, USA
- *Corresponding Author:
- Vincent Robinson, MD
Augusta University
Medical College of Georgia
Division of Cardiology
1120 15th Street, Augusta
Georgia 30912 USA
E-mail: vrobinso@augusta.edu
Accepted on January 19, 2018
Citation: Kapuku GK, Krothapalli SM, Corley JH, et al. Role of BMI and venous resistance in modulation of cardiac filling. Arch Gen Intern Med. 2018;2(1):26-31.
Abstract
Objective: Through our experiment studying the effect of supine exercise on conduit veins, we attempt to investigate the physiology of venous return, cardiac filling, and its relationship to BMI.
Design: Uncontrolled longitudinal study. Setting: Single urban academic medical center.
Subjects: Adult subjects free of cardiovascular disease, and without a history of abdominal surgery that might interfere with CT scan measurements of IVC size and no contraindications to performing near-maximal levels of exercise. Interventions: we studied 14 healthy subjects at supine rest, legs elevated and incremental levels of supine exercise. We calculated an index of venous resistance (VR), and measured aortic and IVC cross-sectional areas (CSA), transmitral filling parameters, and IVC ellipticity. The participants were dichotomized based on BMI: normal BMI ≤ 25 (n=6) and elevated BMI >25 (n=8).
Measurements and main results: Measurements included: Hemodynamics, Transthoracic mitral inflow, Computed Tomography and BMI data. There was decreased IVC CSA in the elevated BMI group at each intervention (p=0.007). VR of the elevated BMI group was consistently higher at all interventions with a group effect noted 1.15 ± 0.14 cm H2O.min/L vs. 0.55 ± 0.15 cm H2O. min/L (p=0.011). Ellipticity was lower in the normal BMI group (p=0.032). EVTI during exercise was lower in the elevated BMI group 11.0 ± 0.90 vs. 15.8 ± 1.04 (p=0.004) and also correlated negatively with VR (r=-0.848 p=0.007).
Conclusion: VR and ellipticity are higher; and IVC CSA is lower in the elevated BMI group. This study points to a negative effect of increasing BMI on cardiac filling due to diminutive IVC remodeling and extramural compression.
Keywords
BMI, Venous resistance, Cardiac filling, Venous return, Left-sided inferior vena cava (IVC).
Introduction
The function of the venous system in influencing venous return and cardiac output has not been studied extensively since Guyton’s original experiments, especially in alert conscious humans [1-3]. This has resulted in difficulty in translating veno–cardiac interactions to bedside clinical care. According to Arthur Guyton venous resistance is 19 fold more important for cardiac output than arterial resistance. In clinical settings where patients have increased intra-abdominal pressure (e.g., acute pancreatitis) or have significant abdominal obesity, major effects on venous resistance and cardiac output may occur.
Reduced circumferential area of the inferior vena cava (IVC) suggests inability to accommodate very high IVC flows such as occurring during exercise. Significant changes in ellipticity over normal physiologic functioning suggests no reserve vasodilatory capacity of the IVC. A rapid attainment of complete sphericity indicates increased IVC pressures which are able to mobilize maximal IVC sphericity, counteracting any external compression.
Cardiac filling and the various factors influencing it have been an area of great interest to physiologists since Starling presented the famous Linacre lecture a century ago [4]. These factors are believed to play an important role in the etiology of diastolic dysfunction. Body mass index (BMI) in the obese and in the overweight range has been shown to be consistently linked to an increased risk of diastolic dysfunction [5-12].
We hypothesize a venoconstrictor remodeling effect on the IVC in overweight, sedentary individuals that reduces the reserve capacity needed to modulate venous resistance (VR) during periods of increased cardiac output.
Materials and Methods
IRB approval was obtained from the medical university, in which the study was conducted. Informed consent was obtained from young healthy volunteers (n=14, ages 21-41 y). Subjects were free of any known cardiovascular disease and had no history of abdominal surgery that could interfere with computerized tomographic scan measurements of IVC [13]. Also, subjects had no contraindications to performing near-maximal levels of exercise.
Exercise was performed by all subjects using an exercise cycle ergometer specifically designed for supine exercise (Biodex Medical Systems, New York). This device was mounted over the computerized tomographic imaging bed. The bicycle ergometer had an adjustable resistance to increase workload. The exercise was performed in 3-minute stages to achieve steady state during exercise [14]. Subjects were rapidly titrated to maximum workload by maintaining a constant pedaling rate of 60 revolutions per minute. The resistance was increased to a point where the patient could no longer pedal; this was defined as 100% or maximal exercise. Two levels of exercise were performed by each subject. The first was at 50% of maximal exercise and the second at 90% [15,16].
Hemodynamics
Central venous pressure was recorded using the central line and peripheral venous pressure was recorded by a side hole in the sheath of the same intravenous line. A plastic, single-lumen, central line with a drum cartridge catheter (Venisystems, Abbott, Sligo, Ireland) was placed in each subject via the basilic vein by a trained cardiac anesthetist. Central venous pressure was recorded using the central line and peripheral venous pressure was recorded by a side hole in the sheath of the same intravenous line. Cardiac output was measured by thoracic bioimpedance monitor (IQ system, Renaissance Technologies Inc., Newtown, Pennsylvania). The thoracic bioimpedance monitor utilizes four pairs of mylar electrode strips applied on opposite sides of the neck and chest. The beat to beat stroke volume was computed using the Nyboer equation, as modified by Kubicek [17]. Thoracic bioimpedance has been shown to correlate well both with Fick and thermodilution cardiac output measurements [15]. An index of venous resistance (VR=PVP-CVP/ CO), was calculated at all stages of the protocol.
Computed tomography
A computerized tomographic (CT) image slice was obtained at the predefined sub-hepatic level at baseline, with passive leg elevation in the stirrups, after returning legs to the supine position, and for each level of incremental exercise. CT scanning (High Speed Advantage, General Electric, Schenectady, NY) was performed utilizing one slice to measure inferior vena cava and aortic cross sectional area at the sub-hepatic level. Here, the entire circumference of the inferior vena cava could be identified clearly from the surrounding soft tissue. The CT images were recorded during quiet held expiration to prevent a false increase in inferior vena cava cross sectional area with the valsalva maneuver. The images were analyzed on a PC computer using NIH Imaging Tutor Software. Three separate measurements of the inferior vena cava and aortic cross sectional area were made for each image and their average was calculated. Each image was also read separately by two experienced readers to assess for inter-observer variation. Ellipticity of the IVC was calculated by dividing the major axis of the inferior vena cava by the minor axis [18-20].
Echocardiographic acquisition
Transthoracic mitral inflow Doppler measurements were performed at baseline and immediately after each level of incremental exercise. These measurements were taken within 2-3 minutes after cessation of exercise. The parameters recorded were: peak velocity of early filling (E), peak velocity of late filling (A), E/A velocity ratio, and E wave velocity time integral (EVTI). Tracings of three consecutive cardiac cycles were analyzed and average velocities were calculated.
Experimental protocol
After subjects consented, they were asked to lie on the CT imaging table. The subjects rested for 10-15 minutes in dim lighting then baseline hemodynamic readings were recorded. CT images were then obtained followed by mitral inflow recording as outlined earlier. After a rest period of three minutes, the subject’s legs were then elevated passively utilizing stirrups. Hemodynamic, CT images and echocardiographic readings were recorded. The subject’s legs were then brought down to the supine position and following a rest period of three minutes, identical measurements were again recorded. The measurement protocol was similarly repeated immediately after the cessation of both 50% and 90% maximal exercise using the supine bicycle ergometer. After the study, the subjects were taken from the radiology suite and lines were removed and adequate hemostasis was ensured.
Statistics
All statistical analyses were performed using SAS 9.2 systems package. Statistical significance was accepted at an alpha level of 0.05 unless otherwise noted. Descriptive statistics were calculated for all variables for each condition measured. A mixed model repeated measures ANOVA was performed to examine differences in cardiac related variables. Differences within each BMI group across conditions, as well as differences between BMI groups within conditions, were examined with condition and BMI group considered fixed effects and subject considered a random effect. Post-hoc comparisons were performed by controlling the overall alpha level using a Bonferroni adjustment for comparisons of interest. The Bonferroni-adjusted alpha levels used to assess significance for the post-hoc comparisons were 0.0014 for VR and ellipticity and 0.0042 for the other variables.
Results
Study subjects were classified into two groups based on BMI, eight (BMI>25) and six (BMI ≤ 25). The mean age of the elevated BMI group was 35 years while that of the normal BMI group was 27 years. The mean heights of the elevated and normal BMI groups were 172 and 175 centimeters respectively.
The baseline heart rate, blood pressure (BP), peripheral venous pressure (PVP), central venous pressure (CVP), cardiac output of the two groups are shown in Table 1.
| Measurements | Normal BMI | High BMI | p value | ||
|---|---|---|---|---|---|
| Mean | Std Dev | Mean | Std dev | ||
| HR bpm | 58.66 | 3.72 | 62.87 | 13.54 | 0.4769 |
| SBP mmHg | 101.83 | 12.07 | 114.75 | 6.81 | 0.0253 |
| DBP mmHg | 54.66 | 8.4 | 62.75 | 7.34 | 0.0788 |
| PVP cm H2O | 6.33 | 2.73 | 11.5 | 7.07 | 0.1177 |
| CVP cm H2O | 2.83 | 1.6 | 3.57 | 2.87 | 0.5822 |
| CO L/min | 4.84 | 0.92 | 5.39 | 1.16 | 0.3585 |
| MAP mmHg | 70.39 | 9.5 | 80.08 | 5.91 | 0.0363 |
| IVC mm2 | 443.1 | 121.95 | 343.42 | 267.26 | 0.4153 |
| AO mm2 | 203.97 | 57.05 | 300.07 | 73 | 0.02 |
Table 1. Baseline hemodynamic and morphological characteristics of high and normal BMI groups.
Within the elevated BMI group, there were significant changes noted in the CVP between interventions. CVP was lower at baseline and in supine position compared to passive leg elevation 3.6 ± 0.90 cm H2O vs. 5.9 ± 0.88 cm H2O (p=0.0011) and 3.1 ± 0.98 cm H2O vs. 5.9 ± 0.88 cm H2O (p=0.0008) respectively. CVP increased with exercise but was still significantly lower at 50% exercise than at the legs elevated position 4.3 ± 1.01 cm H2O vs. 5.9 ± 0.88 cm H2O (p=0.0014). A similar trend was noted in the normal BMI group but was not statistically significant.
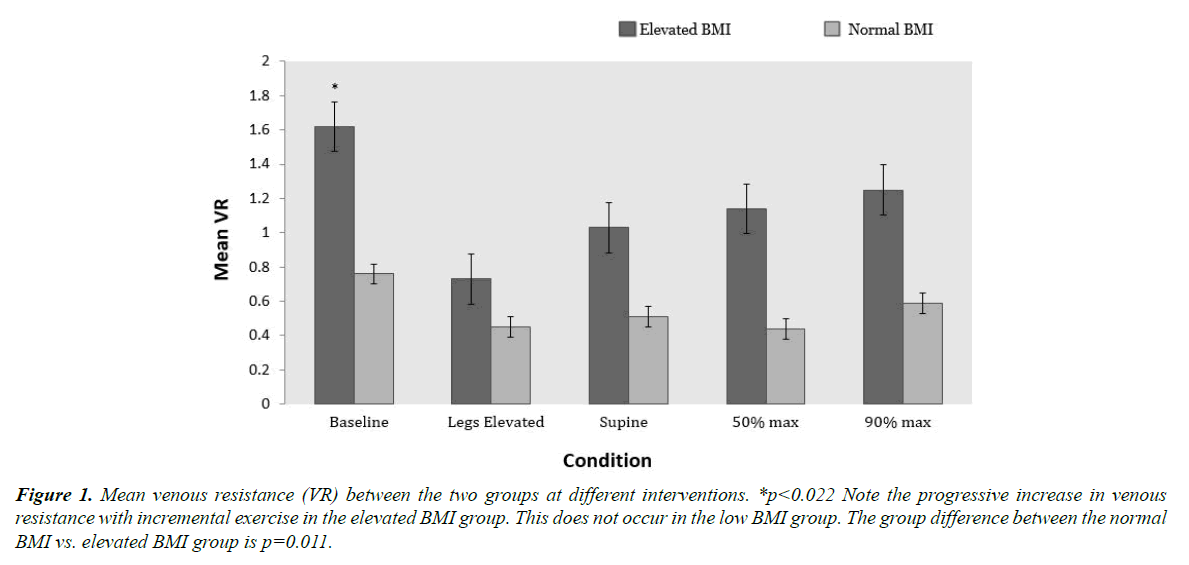
The comparison of VR at each intervention between the aforementioned groups is shown in Figure 1. There was a significant group effect of BMI on the VR between the two groups 1.15 ± 0.14 cm H2O.min/L vs. 0.55 ± 0.15 cm H2O.min/L (p=0.011). The VR of the elevated BMI group was consistently higher than the normal BMI group at all interventions with the greatest difference appearing at baseline. This gap was greatly diminished when the subject’s legs were passively elevated. When the subject was placed back into supine position, it was noted that VR did not return to baseline levels. With 50% and 90% maximal exercise, the VR increased slightly only in the elevated BMI group, but remained much lower than at baseline (Figure 1).
Figure 1. Mean venous resistance (VR) between the two groups at different interventions. *p<0.022 Note the progressive increase in venous resistance with incremental exercise in the elevated BMI group. This does not occur in the low BMI group. The group difference between the normal BMI vs. elevated BMI group is p=0.011.
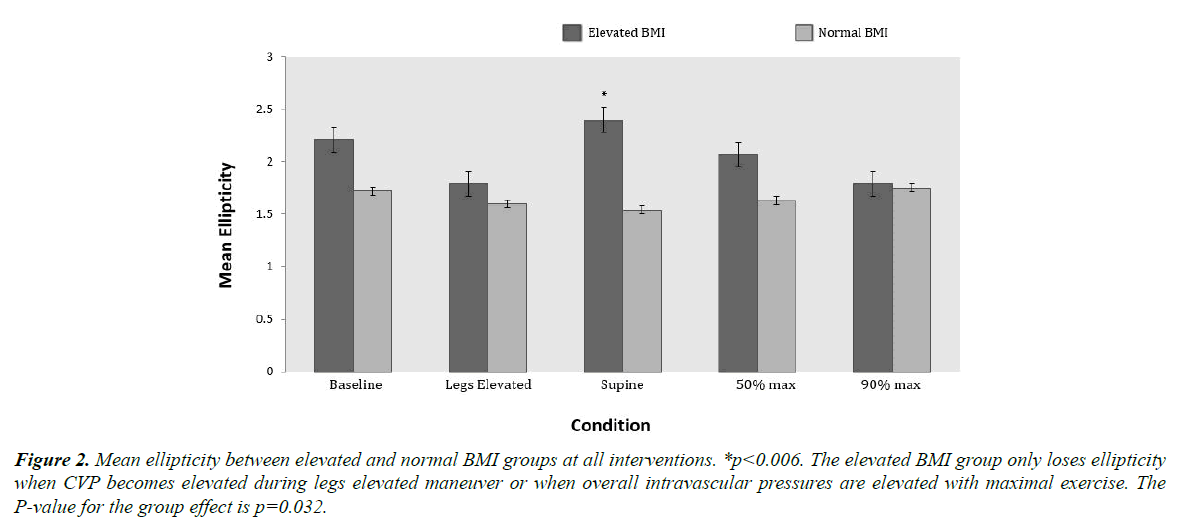
The baseline IVC and aortic cross sectional areas are shown in Table 1. Within the elevated BMI group, the IVC CSA was significantly smaller at baseline and at the supine position than at the legs elevated position 343 ± 77 mm2 vs. 404 ± 83 mm2 (p=0.0024) and 341 ± 69 mm2 vs. 404 ± 83 mm2 (p=0.0039) respectively. Although a similar trend of change in IVC CSA with interventions was noted in the normal BMI group, it did not reach statistical significance. Interestingly, the baseline aortic cross sectional area was significantly larger in the elevated BMI group compared to the normal BMI group of the inferior vena cava was measured at each intervention in the two groups. There was a significant group effect on ellipticity as seen in Figure 2 with the normal BMI group having lower values than the elevated BMI group (p=0.032). Within the elevated BMI group, ellipticity was higher at the supine position when compared to the legs elevated and 90% maximal exercise (p=0.006) and (p=0.017) respectively. With 90% maximal exercise, the ellipticity diminished similar to what was seen with passive leg raising. These changes did not occur in the normal BMI group as the ellipticity remained fairly constant through interventions.
Figure 2. Mean ellipticity between elevated and normal BMI groups at all interventions. *p<0.006. The elevated BMI group only loses ellipticity when CVP becomes elevated during legs elevated maneuver or when overall intravascular pressures are elevated with maximal exercise. The P-value for the group effect is p=0.032.
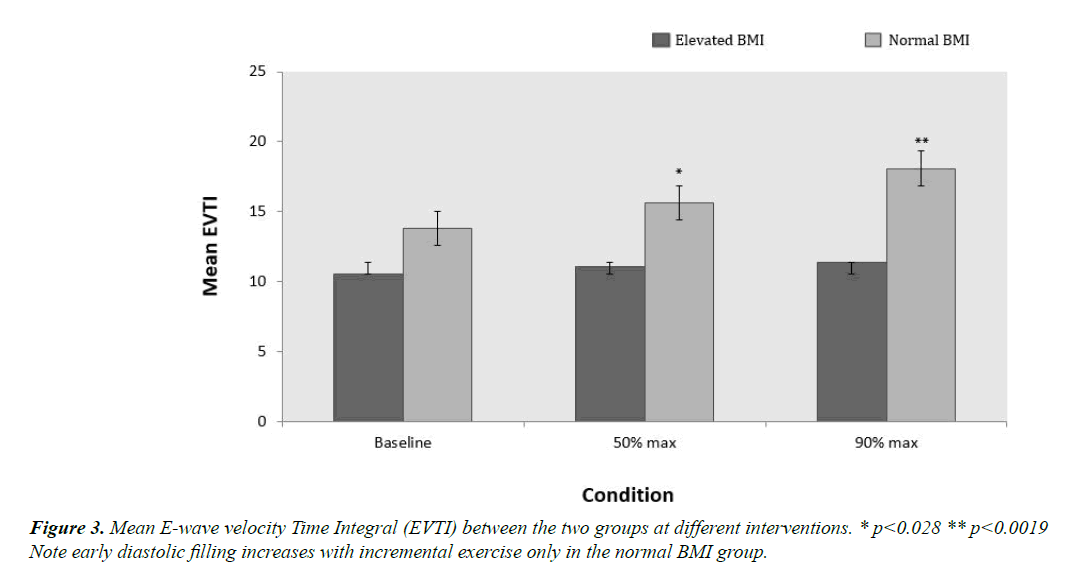
The baseline echocardiographic mitral inflow parameters are shown in Table 2. As seen in Figure 3, EVTI was lower in the elevated BMI group compared to the normal BMI group across all interventions 11 ± 0.90 vs. 16 ± 1.04 (p=0.004). EVTI also showed a considerable increase in the normal BMI group from baseline to 90% max exercise (p=0.046) while staying relatively constant in the elevated BMI group. There was a statistically significant difference in EVTI at both 50% and 90% maximal exercise between the normal and elevated BMI group (p=0.028 and p=0.0019 respectively). There was also a strong negative correlation between EVTI and VR in the elevated BMI group (r= -0.848 p=0.007) but not in the normal BMI group.
| Measurements | Normal BMI | High BMI | p value | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| E cm/sec | 100.63 | 20.96 | 79.88 | 20.33 | 0.086 |
| A cm/sec | 54.81 | 12.27 | 52 | 16.5 | 0.7327 |
| E/A ratio | 1.87 | 0.19 | 1.59 | 0.52 | 0.2361 |
| EVTI cm | 13.81 | 3.87 | 10.52 | 3.64 | 0.2191 |
Table 2. Baseline echocardiographic characteristics of normal and elevated BMI groups.
In the elevated BMI group, A velocity was significantly higher at 50% and 90% max exercise than at baseline (p=0.006 and p=0.0005 respectively). This relationship was not observed in the normal BMI group. E/A was lower in the elevated BMI group compared to the normal BMI group at 50% max exercise (p=0.030). It is also worth noting that within the elevated BMI group, E/A was significantly lower at 50% and 90% max exercise compared to baseline (p=0.027 and p=0.025 respectively).
Discussion
Our findings indicate that higher BMI is associated with increased IVC resistance, higher ellipticity, reduced circumferential area and lower E/A ratio suggesting that increasing BMI has deleterious effect on cardiac filling due to IVC remodeling and extramural compression. Specifically, the study highlights the fact that minor increases in BMI greater than 25, can have significant measurable effects on venous resistance. The ability to mobilize cardiac output with exercise appears to be reduced. This proof of concept study creates the model and methodology for assessing in the future the role of obesity in limiting exercise cardiac output. It also provides a model to assess possible IVC remodeling as part of the training effect, to reduce venous resistance in endurance athletes.
Effect of BMI on venous resistance
From Figure 1, we note a significantly higher VR in the elevated BMI group when compared to the normal BMI group. This difference also parallels IVC morphologies changes due to external compression. The concept of VR and its effect on cardiac filling is crucial to our understanding of how cardiac output is regulated under various physiologic conditions [1,2]. According to the Poiseulle equation (see appendix) the changes in resistance in the IVC are primarily modulated by changes in vessel radius. The resistance in the large veins such as the IVC becomes important in determining preload and cardiac filling because of their function as the most proximal conduit of blood returning to the heart [3,21,22].
We evaluated the dynamics of the venous system through maneuvers such as passive leg raising and varying intensities of exercise. An acute drop in VR was noted in the elevated BMI group with passive leg raising. With this maneuver, a concurrent significant rise in CVP was also noted in this group, suggesting that this increase in intramural pressure may be the mechanism for the drop in VR. In contrast to the elevated BMI group, the normal BMI group tended to have a constant low VR throughout all interventions. This is likely due to the larger IVC sizes seen in this group allowing for increased venous conductance reserve. We also noticed that the normal BMI individuals in our study were very athletic. This is in agreement with the findings of several authors who have reported an increased IVC CSA in endurance athletes with improved venous function with exercise [13,23,24]. Unfortunately, we did not attempt to quantify cardiovascular fitness in the present study.
Unlike the normal BMI group where the VR was fairly constant, the elevated BMI group had a steady rise in VR in response to exercise. This reflects a smaller baseline IVC size with decreased dilatory reserve. The elevated BMI individuals necessitate higher intramural pressures to support the increasing blood flow through the smaller IVC during supine exercise.
Role of extramural compression
A novel measure of collapsible vessel hemodynamics that we analyzed in our study was ellipticity [19,20,25,26]. The differences in ellipticity at baseline and various interventions have allowed us to make further observations. As seen in Figure 2, ellipticity in the elevated BMI group was significantly greater than the normal BMI group across all interventions. The ellipticity in the elevated BMI group varied significantly between interventions. On the contrary, the normal BMI group showed no significant changes in ellipticity between interventions.
The ellipticity of the IVC in the elevated BMI group decreased rapidly with passive elevation of legs, a maneuver, which is expected to increase the intramural pressure of the conduit vein. This increase in the pressure is confirmed by the significant rise in central venous pressure. This increasing intramural pressure with passive leg raising balances the extramural pressure in the elevated BMI group, resulting in the change from elliptical to spherical morphology. The greater ellipticity at baseline as well as the large increase in sphericity with leg elevation (both passively and during exercise) suggests extramural compression as a factor in the elevated BMI group.
The significant differences in morphology between the elevated and normal BMI individuals and its relation to hemodynamics are well explained by the Moreno concept [27,28]. Our observations suggest that the elevated BMI group operates on the vertical portion of the Moreno curve at rest but transitions rapidly to the horizontal portion of the curve during exercise. This changing morphology from elliptical to spherical is presumably due to the increased transmural pressures caused by compression. On the other hand, the normal BMI group maintains a constant mildly elliptical cross sectional area through rest and various interventions. This ability to accommodate increased blood flow without an increase in sphericity is due to lack of external compression and conduit vein dilatory remodeling.
Several authors studying the morphology and hemodynamics of the venous system describe a picture of veins with highly elliptical cross sections which become spherical when intramural pressures are increased [28] However, many fail to clarify that this model of ellipticity does not represent all individuals with varying body habitus.
Relationship among BMI, venous resistance and left ventricular filling
Between the two study groups, there is a decrease in early transmitral flow velocity averaging 30% in the elevated BMI group. This difference increases from baseline to increasing levels of exercise (Figure 3). This relative reduction in early diastolic filling seen in elevated BMI group is also paralleled by a significant progressive increase in late transmitral flow [12,29]. Consequently, a trend of decreasing E/A ratio was observed in the elevated BMI group.
Limitations
One of the limitations of our study is that we measured echocardiographic and computerized tomographic scan measurements immediately after the cessation of exercise (within 60 to 90 seconds) rather than during exercise. This may hypothetically result in attenuation of actual changes in the inferior vena cava dimensions and mitral inflow characteristics, which may have affected our results. We chose to measure these parameters immediately after cessation of exercise because of technical difficulty in measuring mitral inflow velocities while the subject is actively exercising. Supine exercise also relates to a relatively small segment of human exercise spectrum. The technical problems associated with performing exercise in the erect position is the interference and compensation for the effect of gravitational forces on cardiac output, central conduit vein morphology, and the circulatory system in general. Such an interrogation would require different techniques. Nevertheless, our protocol proved suitable to detect exercise induced functional changes in conduit veins and to assess their impact on cardiovascular function. Another possible limitation is that the results could be from underlying differences in blood pressure and not obesity per se. Blood pressure is an arterial phenomenon which affects venous hemodynamics only by degree of sympathetic activation. There is relatively sparse central venous sympathetic innervation, which limits venous constriction in the central veins. We found a minor increase in abdominal aortic cross-section with increasing blood pressure, demonstrating that our methodology was sensitive to the arterial stretch effects of the increase in blood pressure. For these reasons, we do not believe differences in blood pressure, contributed to our IVC morphology and hemodynamic data.
Conclusion
Increasing BMI is accompanied by increased venous resistance and reduced ellipticity of IVC together with early signs of attenuated cardiac filling. This work confirms the important role of conduit vein physiology as link between body habitus and cardiac filling. Weight control may hold promise in interrupting this pathway to deterioration of heart function.
References
- Guyton AC, Lindsey AW, Kaufman BN. Effect of mean circulatory filling pressure and other peripheral circulatory factors on cardiac output. Am J Physiol. 1955;180:463-8.
- Guyton AC, Jones CE, Coleman TG. Cardiac Output and Its Regulation. Circulatory Physiology. 2nd Edition, Saunders, Philadelphia. 1973;pp: 255-62.
- Corno AF. Systemic venous drainage: can we help Newton? Eur J Cardiothoracic Sur. 2007;31(6):1044-51.
- Starling F. The Linacre Lecture on the Law of the Heart. Nature. 1918;101:43.
- Chen YT, Vaccarino V, Williams CS, et al. Risk factors for heart failure in the elderly: a prospective community-based study. Am J Med. 1999;106:605-12.
- He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996-1002.
- Johansson S, Wallander MA, Ruigomez A, et al. Incidence of newly diagnosed heart failure in UK general practice. Eur J Heart Fail. 2001;3:225-31.
- Wilhelmsen L, Rosengren A, Eriksson H, et al. Heart failure in the general population of men: morbidity, risk factors and prognosis. J Intern Med. 2001;249:253-61.
- Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305-13.
- Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775-83.
- Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119(1):44-52.
- Powell BD, Redfield MM, Bybee KA, et al. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol. 2006;98(1):116-20.
- Robinson V, Saleem U, Corley J, et al. Changes in Inferior Vena Cava Caliber from Rest to Exercise and associated Hemodynamics: A Role for Reducing Venous Resistance during Exercise. Circulation. 2010;122:e115.
- Karpman VL. In Cardiovascular System and Physical Exercise, CRC Press, Inc. 1987, Boca Raton, Florida. pp: 135-6.
- Borg GA. Psycho-physical basis of perceived exertion. Med Sci Sports Exerc. 1982;pp: 377-82.
- Fletcher GF, Balady G, Froelicher VF, et al. Exercise Standards: A statement for healthcare professionals from the American Heart Association. Circulation. 1995;91:580-615.
- Ovsyshcher I, Zimlichman R, Katz A, et al. Measurements of cardiac output by impedance cardiography in pacemaker patients at rest: Effects of various atrioventricular delays. J Am Coll Cardiol. 1993;21:761-7.
- Moore FA, Haenel JB, Moore EE. Alternatives to Swan-Ganz cardiac output monitoring. Surgical Clinics of North America. 1991;71(4).
- Berczi V, Molnar A. Non invasive assessment of human large vein diameter, capacity, distensibility and ellipticity in situ: dependence on anatomical location, age, body position and pressure. Eur J Appl Physiol. 2005;95(4):283-9.
- Ribreau C, Naili S, Bonis M, et al. Collapse of thin-walled elliptical tubes for high values of major-to-minor axis ratio. J Biomech Eng. 1993;115(4A):432-40.
- Bressack MA, Raffin TA. Importance of venous return, venous resistance, and mean circulatory pressure in the physiology and management of shock. Chest. 1987;92(5):906-12.
- Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85(7):609-17.
- Goldhammer E, Mesnick N, Abinader EG, et al. Dilated inferior vena cava: a common echocardiographic finding in highly trained elite athletes. J Am Soc Echocardiogr. 1999;12(11):988-93.
- Abergel E, Oblak A. Echocardiography in athletes. Arch Mal Coeur Vaiss. 2006;99(11):969-74.
- Olsen H, Länne T. Reduced venous compliance in lower limbs of aging humans and its importance for capacitance function. Am J Physiol. 1998;275(3):H878-86.
- Shepherd JT, Vanhoutte PM. Veins and their control. WB Saunders, Philadelphia. 1975; pp: 1-269.
- Katz AI, Chen Y, Moreno AH. Flow through a collapsible tube: Experimental analysis and mathematical model. Biophys J. 1969;9(10):1261-79.
- Kresch E, Noordergraaf A. Cross-sectional shape of collapsible tubes. Biophys J. 1972;12(3):274-94.
- Wada H, Shinjo D, Kameda S, et al. Transmitral E/A ratio decreases in association with abdominal fat accumulation in patients with impaired glucose tolerance or mild diabetes without left ventricular hypertrophy. Heart Vessels. 2010;25(1):45-50.