- Biomedical Research (2010) Volume 21, Issue 1
Role of antioxidant vitamin supplementation as a palliative treatment of bone disorders
Alka N. Sontakke*; Sarita A. Shinde, Umesh K. More and Vaishali V. Dhat1Department of Biochemistry,Pad.Dr.D.Y.Patil Medical College Pimpri, Pune, Maharashtra; India
1Department of Biochemistry, Padmashree Dr. D.Y.Patil Medical College; Pimpri, Pune; Maharashtra; India
- *Corresponding Author:
- Alka N. Sontakke
Department of Biochemistry, Pad.Dr.D.Y.Patil Medical College
Pimpri, Pune 411018, India
e-mail : meetans@rediffmail.com
Accepted date: August 13 2009
Abstract
A total of 198 patients of various bone disorders like osteoporosis, chronic renal failure, fractures and bone malignancy attending the OPD between October 2002 to May 2005 at Dr. D.Y. Patil Medical College, Hospital and Research Centre and Command Hospital, Pune were selected for the study. Assessment of the basal serum levels of biochemical markers like Tartarate resistant acid phosphatase (TrACP), Malondialdehyde (MDA), Alkaline phosphatase, free calcium (Ca++), Inorganic phosphorus (Pi), Superoxide dismutase (SOD) and erythrocyte reduced glu-tathione (GSH) was done. Then, the patients were divided into groups A (Vitamin E- Evinal 400 mg), B (Vitamin C- Celin 500 mg) and C (E+C) for administering antioxidants over a period of 90 days. Biochemical markers were reassessed in all the groups after 45 and 90 days of supplementa-tion. The results revealed that after 90 days supplementation there was significant reduction in serum MDA and serum ALP as compared to basal levels (p<0.001) in all three groups. Serum SOD and erythrocyte GSH showed significant increases compared to basal level (p<0.001 ) in all three groups . Serum TrACP was significantly reduced in group A and B (p<0.001) as compared to basal levels. Serum free calcium and phosphorus were unaltered. To conclude antioxidant vitamins E and C individually or conjointly improve bone meta-bolic status in skeletal disorders.
Keywords
Oxidative stress, Osteoblastic and Osteoclastic markers, Bone disorders, vitamin E and C
Introduction
In bone disorders there is imbalance of osteoclastic and osteoblastic activity leading to overproduction of free radicals like superoxide [1]. Normally they act as chisels and are responsible for bone remodeling [2]. The free radicals though a cutting tool, their destructive action is reconciled with discrimination, and thus controlled pro-duction of free radicals is probably the key to make this destructive activity to complement the construction ef-fected by osteoblasts. Thus the correct blend of the two leads to the ultimate goal of remodeling [3,4,5].
These free radicals if produced in excess, in turn, are ex-pected to produce oxidative stress mainly through lipid peroxidation. A pilot study which was conducted in our institute has, precisely corroborated this expectation. Thus the osteoblastic activity is likely to be eclipsed by osteo-clastic activity in all bone disorders namely osteoporosis, renal osteodystrophy, bone malignancies and fracture [1]. The natural correction of the imbalance to restore the con-struction and to ameliorate the condition obviously cannot cope with the onslaught of oxidative stress. Thus external intervention by way of supplementation of antioxidants to beef-up whatever natural metabolic effort, seems in order without which the system may succumb to oxidative stress.
In view of this the present study was undertaken to assess the plausible role of antioxidant vitamins in bone disorders.
Materials and Methods
Study design
The study group included 198 clinically diagnosed cases of skeletal disorders like osteoporosis (n=75), chronic renal failure (n=32), fractures (n=33) and bone malignan-cies (n=58). Various bone disorders were considered to minimize biased results due to single skeletal pathology. This was done as the study design was to assess effect of antioxidant vitamin supplementation on deranged bone metabolism of skeletal pathology in general.
The patient selected were those attending the OPD at Dr. D Y Patil Medical College, Hospital and Research Centre and Malignant disease training centre, Command Hospi-tal, Pune from October 2002 to May 2005. The patients with bone disorders were in the age group between 25-70 years. Patients suffering from non-osteoid metabolic dis-orders like Diabetes Mellitus, Hyperparathyroidism, those on antioxidant supplementation or anti-resorptive therapy and smokers were excluded. Fifty age and sex matched healthy controls were selected for comparison of altered bone metabolism in skeletal disorders. The controls were not suffering from any disease and were not on any medi-cation or vitamin supplementation before and during the study period. All patients and controls gave informed consent for the study.
Analytical Methods
Fasting venous sample (10 ml whole blood) was col-lected, under aseptic conditions, from both the control and study groups around 8.00am. 6 ml of it was collected in a plain sterile bulb for the estimation of ALP; Pi, SOD and MDA
100 μL serum was kept for the measurement of TrACP. 3 ml of whole blood was collected anaerobically in a capped conical centrifuge tube for the free calcium esti-mation. 1 ml of whole blood was collected in the acid citrate dextrose (ACD) bulb for the erythrocyte GSH es-timation.
Samples were collected and analyzed for bio-chemical markers within 3 hours of sample collec-tion by the following methodologies: SOD by Marklund and Marklund [6], MDA-Wilbur et al [7], ALP [8], Pi [9], TrACP [10], Ca++ by ion selective electrode [11] and Erythrocyte GSH-Beutler et al [12].
After assessment of baseline concentrations of biochemi-cal markers, the test group was subdivided into three groups A, B and C randomly but maintaining the ratio of various disorders in each group. Group A was adminis-tered only Vitamin E (Evinal 400 mg), Group B were ad-ministered only Vitamin C (Celin 500 mg). Group C were received conjoint therapy of Vitamin (E+C) i.e. for a pe-riod of 90 days. Samples were collected and analyzed at 45 and 90 days after supplementation.
Statistical analysis
The sample size was decided in consultation with statistian. The results were expressed as mean ±S.D. Compari-son of control and test group was done by unpaired ‘t’ test. The change in markers before and after antioxidant supplementation was studied by paired ‘t’ test. Compari-son of efficacy of antioxidant supplementation was tested by ANOVA.
Results
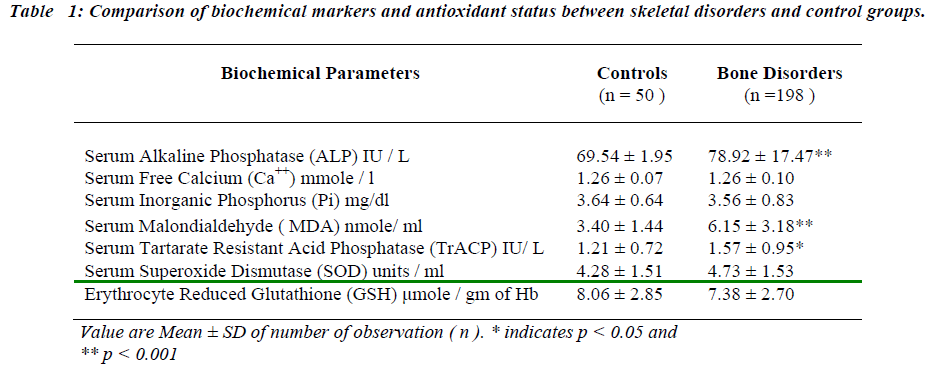
The present study involved the comparison of alteration in the biochemical markers in the skeletal disorders with healthy controls (Table I). The results revealed significant increase in baseline levels of serum MDA, TrACP and ALP in bone disorders as compared with controls (p < 0.001, p < 0.05, p<0.001 respectively) No statistical dif-ference was observed in concentration of serum Ca++, Pi, SOD and erythrocyte GSH.
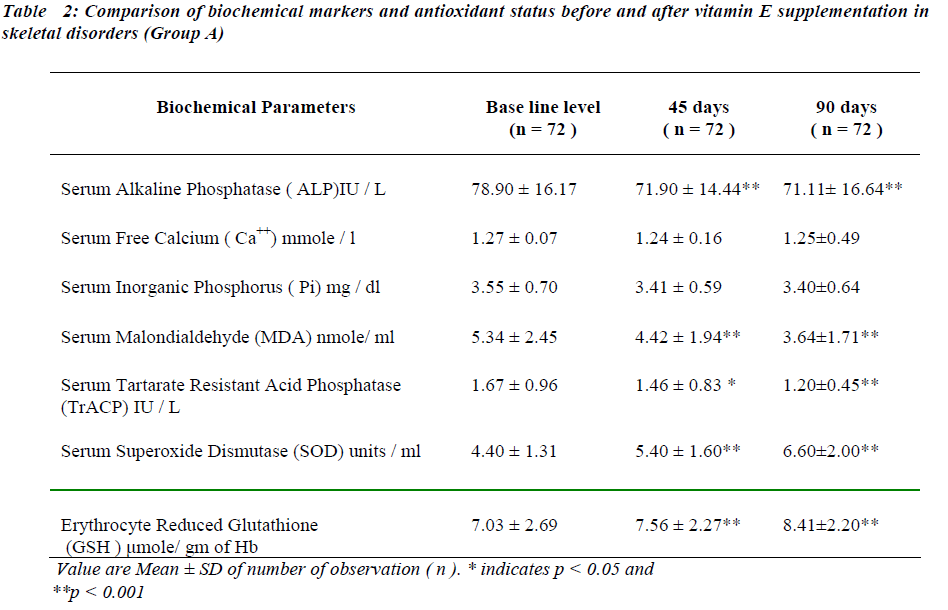
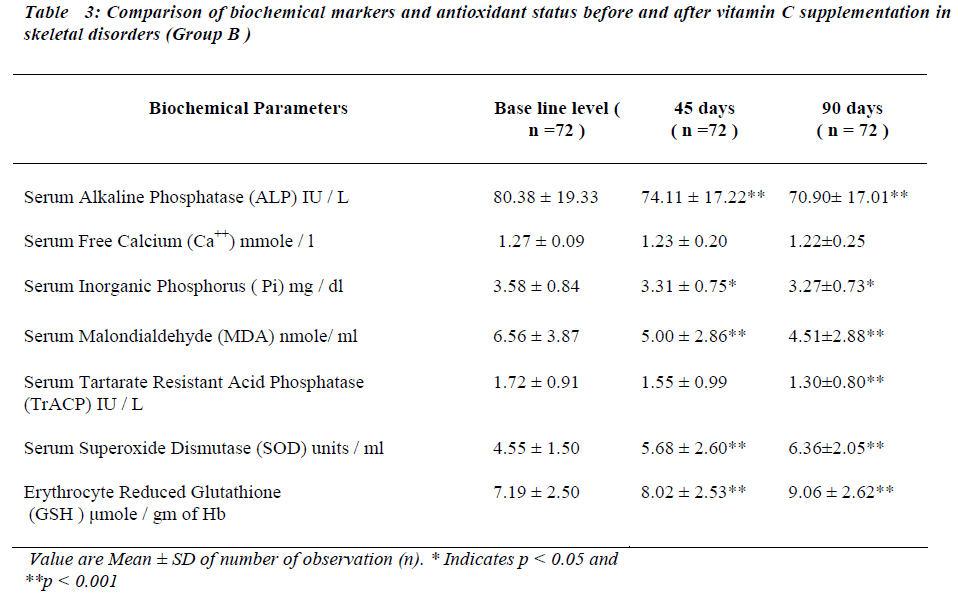
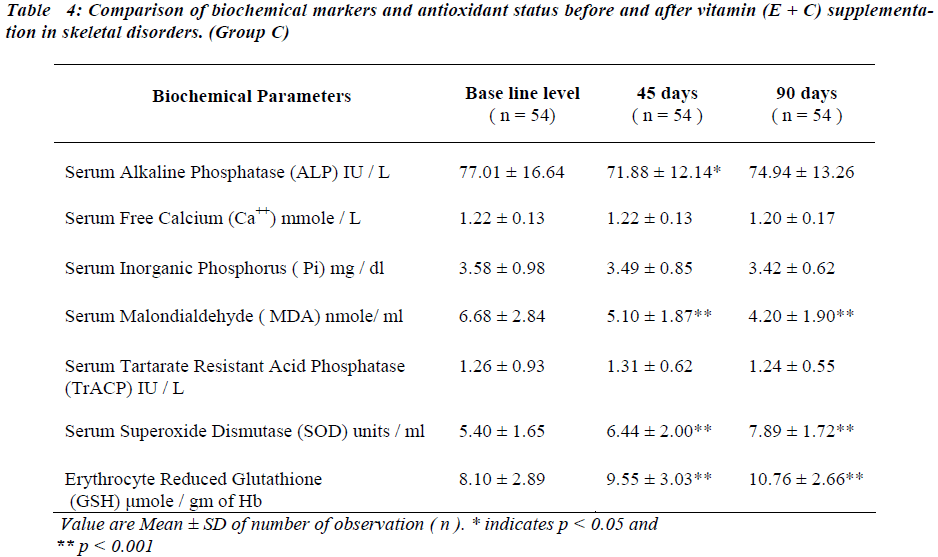
After administration of antioxidants individually and con-jointly to the test group for a period of 45 days, serum MDA is significantly reduced (p < 0.001). No significant change was seen in serum TrACP levels (Tables II,III and IV ).
There is significant decreases in serum ALP levels (p < 0.001) while no significant change is seen in serum free Ca++ after 45 days of antioxidant supplementation in any of the group (Table II, III and IV). Serum Pi is signifi-cantly reduced (p < 0.05) only in group B (Table III) while no significant change was seen in group A and group C (Table II and IV respectively).
Serum SOD and erythrocyte GSH levels are significantly increased in all the groups after 45 days of antioxidant supplementation (Table II, III and IV).
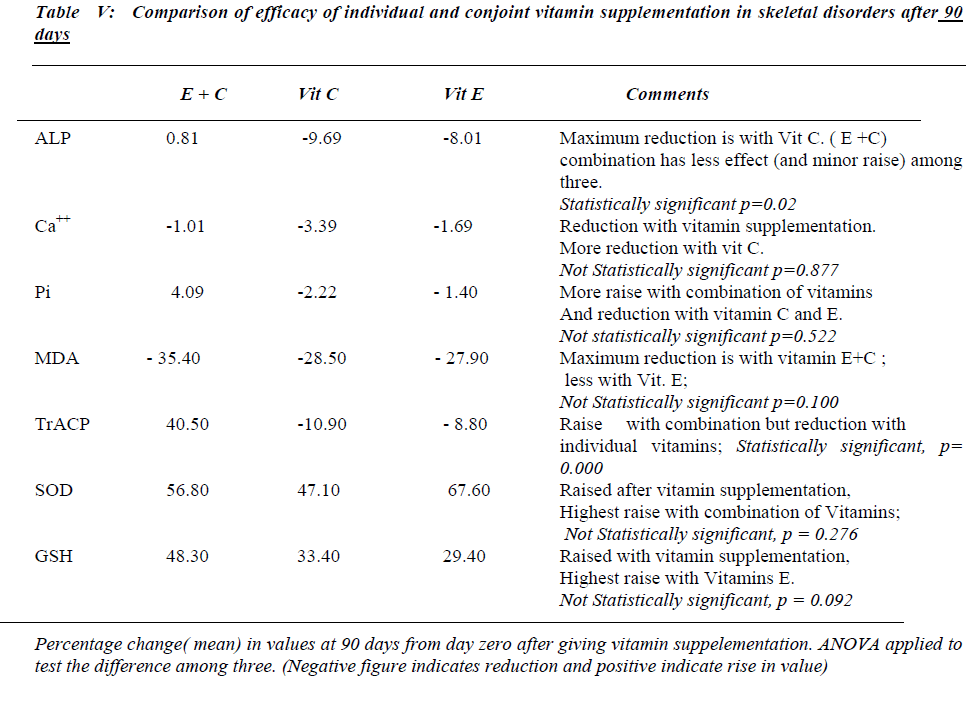
Serum MDA levels continue to decrease significantly ( p<0.001) and serum TrACP levels are significantly re-duced in group A and B (Table II,III) after 90 days of antioxidant supplementation.
Serum free Ca++ and Pi remain unaltered in all groups and serum alkaline phosphatase levels continued to decrease at 90 days in group A and B. Serum SOD and erythrocyte GSH levels continue to increase significantly after 90 days in all the groups.
Referring to Table No IV number of subjects in group C differs from group A and B due to drop out of cases for subsequent follow up.
Discussion
The skeletal disorders are associated with complex inter-play of osteoclastic and osteoblastic activities. The pilot study of this project revealed increased oxidative stress in skeletal disorders [1,2]. The role of antioxidants in con-trolling free radical formation and hence alleviating oxi-dative stress is well established [13,14]. The present study, hence, was an attempt to assess role of antioxi-dants, vitamin E and C in reducing oxidative stress in cer-tain skeletal disorders. Biochemical markers of bone me-tabolism used for assessing the response of the antioxi-dants included osteoblastic and osteoclastic markers [15,16]. The correlation of the markers with antioxidant status was also studied. The mean values of all the parameters in the control group were found to be in agreement with the quoted mean values obtained by other workers [6-12].
In the skeletal disorders under consideration, all bio-chemical markers are increased except serum free Ca++ and Pi. Serum SOD and erythrocyte GSH remain unal-tered as compared to their healthy counterparts. The rise in serum MDA may be due to overwhelming of osteoblas-tic activity by osteoclastic activity due to activation of osteoclasts. They use a variety of chemical agents to de-grade bone. One important component is generation of superoxide radicals [3,4,5,17]. This study reveals no change in basal values of serum SOD and erythrocyte GSH concentration in skeletal disorders, which may ex-plain rise in serum MDA levels, occurring due to in-creased lipid peroxidation as a result of increased produc-tion of superoxide by osteoclasts.
Osteoclasts being active during the process of bone re-sorption, secrete large quantities of TrACP in resorption lacunae leading to the rise in TrACP levels in skeletal disorders [18].
After antioxidant supplementation for 45 days there seems to be improvement in the lipid peroxidation index i.e. fall in serum MDA indicating inhibition of lipid per-oxidation. Vitamin E exerts it′s antioxidant property by preventing chain propagation [13,19]. Vitamin C acts as water soluble antioxidant by inhibiting initiation of lipid peroxidation13. The net result is reduction in lipid peroxi-dation resulting in reduced serum MDA levels. Serum TrACP concentration is not altered in the patients of skeletal disorders in different groups of supplementation.
The osteoblastic markers serum free Ca++ and serum Pi do not show significant alterations after 45 days of antioxi-dant supplementation. Serum ALP is significantly re-duced. This finding differs from the observations of other workers who have reported that osteoclastic markers re-spond earlier than osteoblastic markers when the bone resorption is inhibited [16,20,21]. Hence the findings of the study indicate that both osteoclastic and osteoblastic activities together improve the bone metabolic status.
In skeletal disorders the antioxidant status is improved in all the groups of vitamin supplementation, which explains the decrease in MDA levels after 45 days of supplementa-tion. Rise in serum SOD is seen in concordance with find-ing of other workers [19].
After 90 days of antioxidants supplementation, further significant fall in concentration of osteoclastic marker i.e. TrACP , lipid peroxidation index i.e. serum MDA and osteoblastic marker serum ALP along with rise in anti-oxidant status i.e. serum SOD and erythrocyte GSH is observed. Vitamin E in addition to inhibition of lipid per-oxidation enhances the bioactivity of nitric oxide which is interrelated with raised serum SOD levels [3,21,22]. This may be responsible for the decrease in the serum TrACP levels as a reflection of the inhibition of the osteoclastic activity. Vitamin C improves the antioxidant status by i) increasing erythrocyte GSH levels by being a cofator for NADP reductase. This enzyme plays a key role in the regeneration of GSH from oxidized glutathione. ii) Re-generating alpha tocopherol [23,24].
Serum free Ca++ and serum Pi remain unaltered indicating that basically in skeletal disorders an imbalance is seen in osteoclastic and osteoblastic activity rather than defect in mineralization due to calcium and phosphorus availability [25].
Conclusion
The study revealed an improvement in the osteoclastic and osteoblastic parameters and hence the bone metabolic status in skeletal disorders after antioxidant vitamin sup-plementation. Referring to the efficacy of antioxidant vi-tamins, both E and C supplemented individually or con-jointly are equally effective in improving the bone meta-bolic status in skeletal disorders. The observations of the study also suggest that duration of antioxidant supplemen-tation has a strong bearing on the response of the bio-chemical markers and antioxidant status. Prolonged sup-plementation is found to be beneficial in improving the bone metabolic status. Hence vitamin E and / or C sup-plementation may prove to be cost effective in palliative treatment of skeletal disorders.
Acknowledgements
We acknowledge the financial assistance given by ICMR, failing which the publication of this paper would not have been possible. We also acknowledge the cooperation of the departments of Biochemistry, Medicine, Obstetrics & Gynecology and Orthopedics, Dr. D. Y. Patil Medical College; Pune and Malignant disease training centre, Command Hospital, Pune in completing this project.
References
- Sontakke AN, Tare RS. A duality in the roles of reac-tive oxygen species with respect to bone metabolism. Clinica Chimica Acta 2002; 318: 145-148.
- Victoria L Contie. The sculpting of bone; NCRR Repo-rter, Summer 2000; Cover story 1-4.
- Karten T, Colein-Osdoby P, Patel N. Potentiation of osteoclast bone resorptive activity by inhibition of ni-tric oxide synthase. Proc Natl Acad Sci USA 1994; 91: 3569-3573.
- Silverton SF, Mesaros S. Osteoclast radical interac-tions: NADPH causes pulasatile release of NO and sti-mulates superoxide production. Endocrinology; 1975; 136:5244-5247.
- Yang S, Ries WL and Key Jr. NADP oxidase in the formation of superoxide in osteoclasts. Calcific Tissue Int (1998); 63: 346-350.
- Marklund S, Marklund G. A simple assay for superox-ide dismutase using auto oxidation of pyrogallol. Eur J. Biochem 1974; 47: 469-472.
- Wilbur KM, Bernheim F, Shapiro OW. The thiobarbi-turic acid method for malondialdehyde estimation. Arch Biochem Biophys 1943; 250: 305-313.
- BurtisCA, Ashwood ER. Teitz textbook of Clinical Chemistry, 3rd Ed. W.B. Saunders Co Philadelphia PA 1999; 1351-1352.
- Fiske CH, Subarrow Y. Harold Varely’s. Practical Clinical biochemistry, 4th Ed. Delhi, India: CBS Pub-lishers and Distributers 1975; 446-447.
- Plebani M, Bernardi D, Zaminotto M. New and tradi-tional serum markers of bone Metabolism in the detec-tion of skeletal metastasis : Clinical biochemistry 1996; 29 (2); 67-72.
- Bowers GN, Jr Brassad C, Sena SF. Measurement of ionized calcium levels in serum with ion selective elec-trodes. A mature technology that can meet the daily service needs. Clinical Chem 1986); 32: 1437-1444.
- Buetler E, Duron, O Kelly B M. Improved method for the determination of blood glutathione. J Lab Clin Med(1963), 61: 882-888.
- Frei. ROS and Antioxidant vitamin: Mechanism of action. Am J Med 1994; 97: 3A-7S.
- Patrick H. “Say no to arthritis”. The proven drug free approach to arthritis, 1st edition; Pub. ION Press. (1993)
- Claude DA. Osteoporosis: Using ‘bone markers’ for diagnosis and monitoring. Geriatrics (1995);.51(4); 24-29.
- Eastell R, Blumsohn A. The value of biochemical markers of bone turnover in osteoporosis. The journal of Rheumatology 1997; 24(6); 1215-1217.
- Yang S, Ries WLLL. Superoxide generation in translo-cated β-lymphocytes from patients with severe, malig-nant osteoporosis. Molecular and cellular biochemistry; 1999; 199:15-24.
- Miller SC. The rapid appearance of acid phosphatase activity at the developing ruffled border of parathyroid hormone activated medullary bone osteoclasts. Calcific Tissue Int 1985; 37:526-529.
- Jayakumari N, Gopi. Efficacy of Vit E supplementation on lipid peroxidation and antioxidant enzymes in pa-tients with Coronary Artery Disease, Biomedicine 1998; 18 (3); 137-143.
- Garnero P, Shih WJ, Gineyts E. Comparison of new biochemical markers of bone turnover in late post menopausal osteoporotic women in response to alen-dronate treatment. J Clin Endocrinol Metab 1994; 79: 1693-1700.
- Laurence M Demers. Recent advances in biochemical markers of bone turnover. Clinical Chemistry 1994; 40 (11); 1664-1665.
- John F Keaney, Jr Daniel I Simen. Vitamin E and vas-cular homeostasis implication for atherosclerosis. The FASEB Journal 1999; 13:965-975.
- Murray RK, Keeley FW. Micronutrients and vitamins In: Murray RK, Granner DK, Mayes PA, Rodwell VW, Harper’s illustrated biochemistry, 27th ed; Lange medi-cal Books, Stamford, Connecticut Mc Graw hill (2006): 494.
- Kasper DL, Braunwald E, Fausi AS Nutritional re-quirement and dietary assessment in Harrison’s princi-ples of internal medicine, 16th Ed ,McGraw-Hill pub-lication 2005, I ; 408.
- Frederick RS. Metabolic bone disease: renal osteodys-trophy, osteoporosis. In: Felig P, Baxter JD, Broadas AE, Frohman AL, editors. Endocrinology and metabo-lism 2nd Ed. New York: McGraw-Hill; 1987:1478-1480.