- Biomedical Research (2012) Volume 23, Issue 1
Review: Lactococcus Lactis: An efficient Gram positive cell factory for the production and secretion of recombinant protein
Roshan D’Souza, Dipendra Raj Pandeya*, Seong-Tshool Hong.
1Laboratory of genetics, Department of Microbiology and Immunology, Institute of Medical Science, Chonbuk National University School, Chonju, Chonbuk 561-712,South Korea
2Department of Biochemistry, Nepal Army Institute of Health Sciences, Sanobharayng, Kathmandu, Nepal
- Corresponding Author:
- Seong-Tshool Hong
Laboratory of Genetics,
Department of Microbiology and Immunology
Institute of Medical Science
Chonbuk National University School, Chonju, Chonbuk
561-712, South Korea.
Accepted date: July 06 2011
Abstract
Lactococcus lactis, a model lactic acid bacterium that is widely used in the dairy industry, which proves beneficial due to its easy protein secretion and purification. It is also a potential host for the production of therapeutic recombinant proteins. Many heterologous proteins have been produced in L.lactis but very few report the whole system of the gene expression machinery and its application. Here, we review the complete gene expression system for the L.lactis right from the promoters, roll of signal peptides, site of expression and specific protein targeting system. Thus the review can be a guide for the appropriate selection of strains and site for the expression system in L.lactis.
Keywords
Lactococcus lactis, therapeutic recombinant proteins, Gene expression
Introduction
Lactic acid bacteria (LAB) are gram positive bacteria that include Lactococci, streptococci, and lactobacilli, all of which have long been used as starters for food fermentations [1]. Many of these LAB are associated with traditional and industrial production of fermented food, beverages and animal food. Besides providing natural benefits like exerting the positive action in lactoseintolerant consumers by providing lactase in the gut, it’s been also proved beneficial in delivering the digestive enzymes to supplement pancreatic deficiency in humans.
There have been numerous efforts made since three decades, to study and understand the genetic basis of the Lactic acid bacteria’s properties in order to obtain the better industrial yield. Lactococcus lactis, becoming the most suitable expression cell factory these days for the heterologous protein secretion. This organism is easy to handle and, with improved understanding on the genetic level, the last couple of decades has resulted in the development of a number of gene expression systems based on L.lactis [2,3]. Many genetic tools have been developed and its complete genome was recently sequenced [4], which makes it easier for researchers worldwide to work its gene manipulations. There have been numerous heterologous proteins like reporter molecule, bacterial, eukaryotic, viral antigens, Interleukins, allergens, virulence factors, bacteriocins and enzymes [5].
Huge success has been noticed in targeting the protein secretion to cytoplasm, cell wall and also to extracellular medium. L.lactis being the non pathogenic food grade bacteria shows much efficacy as live antigen and enzyme carriers, thus it proves beneficial for the oral administration than the attenuated pathogenic microorganisms like salmonella typhi and chlorella [6,7].And also LAB is able to survive through the GIT of human beings and other animals, with a retention time of 2-3 days in gut, but it does not invade or colonize the mucous and does not evoke strong host immune responses(8).
This decade has been significant advances in the genetic study of LAB resulting in the development of greater number of genetic techniques, transformation protocols, and sophisticated vector, integration and amplification systems. In this review we describe the new advances and approaches concerned with the L.lactis being used as the live delivery vector.
Gene expression systems
High level production of heterologous proteins in L.lactis has been obtained using lactococcal constitutive or inducible promoters. The approach of the usage of either inducible or constitutive promoters depends upon the primary goal of the experiment. A lot of expression systems have been already described in L.lactis [9,10]. In this following section we mainly focus upon the promoters which have been proved beneficial for the numerous heterologous proteins.
Constitutive promoters:
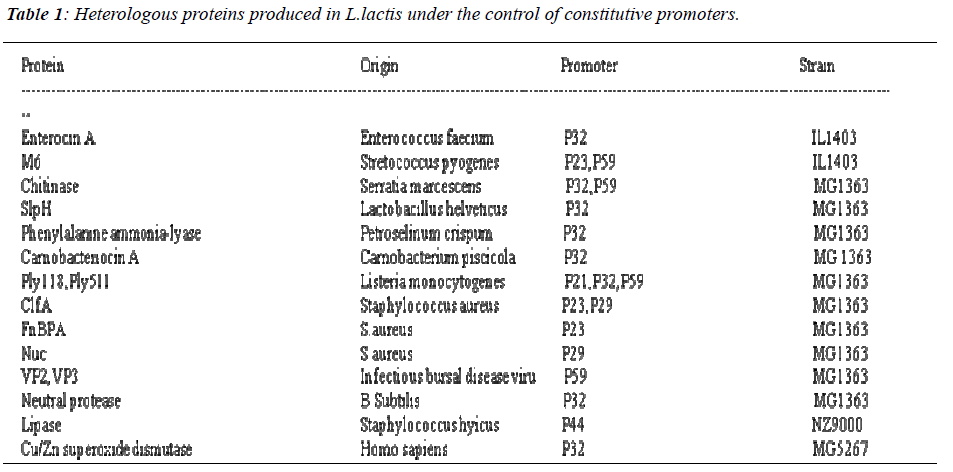
In L.lactis, several constitutive promoters have been identified which are not controlled by regulatory or growth conditions. Numerous strategies have been followed to isolate promoters from L.lactis and other LAB[10)].Out of which the strategy based on screening vectors ,both plasmids and transposons, carrying the promoterless reporter genes such as those encoding chloramphenicol resistance or beta galactosidase or betaglucuronidase have been followed. P21, P23, P32, P44 and P59 are reported to be efficient promoters in L.lactis (Table.1). Depending upon the chloramphenicol acetyl transferase activity levels these promoters have been distinguished into strong (P21 P23, P59) and wea k (P32,P44) [11].Study on constitutive promoters have been still under progress and numerous research is going on. However, inducible promoters proved to be better promoters in industrial field.
Inducible promoters:
Significant studies have been done on inducible promoters. These promoters express proteins when there is stimulation from the environment. Many L.lactis promoters are known to be inducible by stress conditions such as phage attack, thermal or pH shift, or by a specific sugar [10].
Sugar inducible expression systems: Most genes involved in sugar transport and catabolism are organized into operons that are strongly expressed and controlled at the level of transcription initiation. The best characterized lactococcal promoter is that of L.lactis operon, which is controlled by the autoregulated LacR repressor. The induction my lactose is effected by the intermediate tagatose-6-phosphate that inactivates the LacR repressor. This system has been reported to have the highest efficiency of all sugar inducible expression [12,13].
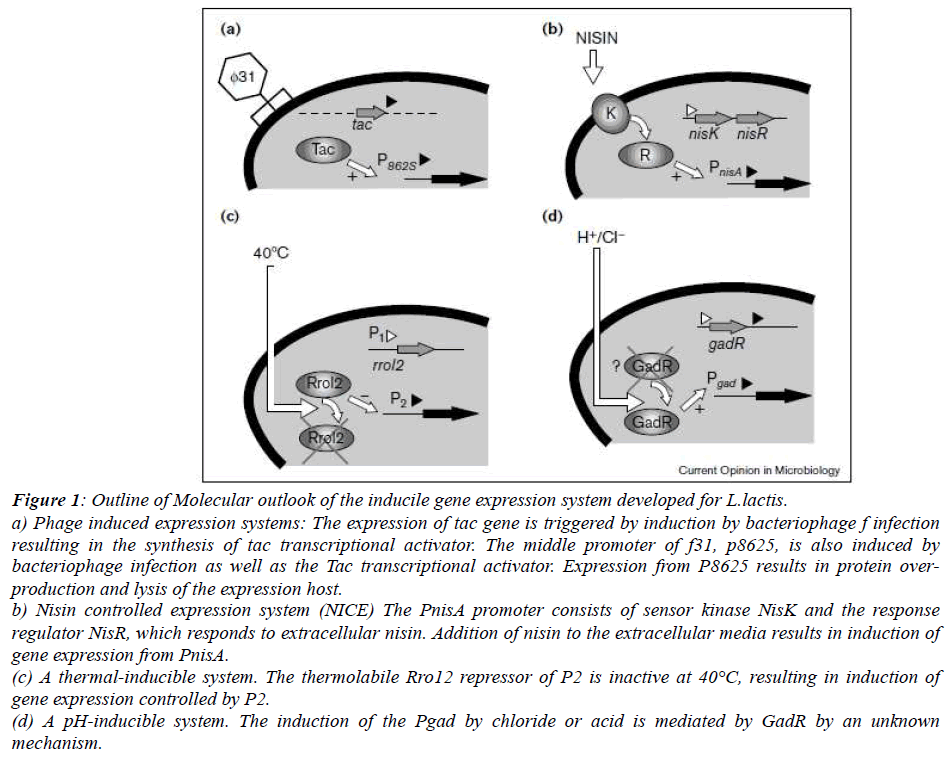
Phage induced expression systems: Infection from the bacteriophage ?31 results in the lysis of the production strain. Using as expression plasmid containing the replicon origin of ?31 that is also induced upon bacteriophage infection which leads to massive expression (Fig.1.a) [14].
Thermal and pH induction: Thermolabile P1 and P2 promoters control the expression of Rro gene, encoding the repressor and the tec gene respectively. Thermolabile variant Rro repressor, Rro12, that upon shifting from permissive growth temperature of 24°C to 42°C resulted in approximately 500 fold induction which is controlled by P2(Fig.1.c) [15]. Acidification being the one of the important properties of LAB, several promoters have been uncovered which gets expressed in the pH shift (Fig.1.d) [16). There have been two promoter systems which is studies extensively. Firstly, gad promoter driving the expression of the gad CB operon predicted to encode a glutamate-gamma-amino butyrate antiporter and glutamate decarboxylase, respectively, which operate in glutamate dependent acid stress resistance and one more promoter p170,being the natural promoter of uncharacterized gene termed orfX, is induced by the pH decreease. P170 expression system offers the major advantage of self inducibility via acid accumulation in the medium during growth [17].
Nisin controlled expression system (NICE): The NICE system has been derived from the molecular characterization of the production of nisin, a post-translationally modified antimicrobial peptide, produced by several strains of L. lactis that are widely used in food industry [18]. NICE comprises of regulatory elements of the nis operon:PnisA, the nisin inducible promoter and nisRK, the regulator-sensor 2- component system (Fig.1.c). Nisin system has been extensively used in the industrial production because of its easy use, high protein yield and and large scale production process. However, nisin addition is costly and during protein production, further purification is needed.
P(Zn)zitR expression system: This has been recently developed, which controls expression of the zit operons, zit is putatively involved in Zn2+ uptake by the ABC transporter zitSQP, and it is controlled by zitR, and MarR family transcriptional regulator. Thus the P(Zn)zitR controlled expression proved to be tightly regulated in response to zinc concentration in the medium[19].
Roll of signal peptides
Most proteins are secreted via the Sec pathway are synthesized as precursors containing the mature protein and N-terminal signal peptides(SP) which directs the protein into different target like certain organelles such as the nucleus, mitochondrial matrix, endoplasmic reticulum, chloroplast, apoplast and peroxisome. Specific organelle transfer depends upon the length and charge of the signal peptide. Signal peptides also retards the precursor folding, together with the action of specific chaperones [20]. Once the peptides reach the relevant organelles they get cleaved by signal peptidase. Although the primary sequences are poorly conserved, all SP’s display a common tripartite structure including a positively charged N terminus hydrophobic core, and a neutral or negatively charged C terminus containing the SP cleavage site [21].
Figure 1: Outline of Molecular outlook of the inducile gene expression system developed for L.lactis. a) Phage induced expression systems: The expression of tac gene is triggered by induction by bacteriophage f infection resulting in the synthesis of tac transcriptional activator. The middle promoter of f31, p8625, is also induced by bacteriophage infection as well as the Tac transcriptional activator. Expression from P8625 results in protein overproduction and lysis of the expression host.
b) Nisin controlled expression system (NICE) The PnisA promoter consists of sensor kinase NisK and the response regulator NisR, which responds to extracellular nisin. Addition of nisin to the extracellular media results in induction of gene expression from PnisA.
(c) A thermal-inducible system. The thermolabile Rro12 repressor of P2 is inactive at 40°C, resulting in induction of gene expression controlled by P2.
(d) A pH-inducible system. The induction of the Pgad by chloride or acid is mediated by GadR by an unknown mechanism.
Numerous L.lactis signal peptides have been identified and characterized. In this review we have focused upon three main signal peptides which have been used and proved to be efficient. The structure of a typical SP includes three distinct regions: (i) an N-terminal region (n-region) that contains a number of positively charged amino acids (lysines and arginines); (ii) a central hydrophobic core region (h-region); and (iii) a hydrophilic cleavage region (c-region) that contains the sequence motif recognized by the signal peptidase. Despite structural similarities, large sequence variations occur in SP’s [22,23].
SPnuc: SPnuc is atypical, as it is 60 residues and contains two hydrophobic stretches that may form a hairpin in the cytoplasmic membrane during translocation. Previously Nuc was used as secretion reporter to assess the secretion capacity of L.lactis [24]which is small and stable secreted protein, is generally and biochemically well characterized. Further information can be obtained on Simonen et.al [37].
SP310: This is the natural signal peptide, which shows sub minimal secretion compared with other signal peptides. However, Site directed mutagenesis was carried out to introduce mutations in one or more of the three domains present in the SP310.several changes in the cleavage in the cleavage region improved Nuc secretion efficiency. SP310mut2 showed increased efficiency in both simple flask cultures and in fermenter cultures with high production levels [25].
SPExp4: The structure and amino acid composition best fit with the gram positive consensus and this has been proved to efficiently drive the secretion of the murine IL- 12p40 sub-unit in L.lactis [26].
SPUsp45: This signal peptide has been is most commonly used for heterologous protein secretion in L.lactis. This has been combined with either a constitutive promoter like p59 [27] or the inducible NICE system [28, 29, 30, 31] . The usp45 gene encodes the major extracellular protein from L.lactis. The deduced sequence of the 27 residue leader peptide revealed the tripartite characteristics of a signal peptide. This leader peptide directed the efficient secretion of the homologous proteinase (PrtP) in L. lactis, indicating that the putative signal peptide of PrtP can be replaced by the 27 residue Usp45 leader peptide [26].
Roll of synthetic propeptide: A short synthetic propeptide( LEISSTCDA) can be fused between a signal peptide and mature part of the protein to improve the efficiency of heterologous secretion in L.lactis. The probable negatively charged residues of the propeptide optimize the charge balance around the transmembrane domain of the signal peptide and thus enhance its efficiency and protein translocation [24,32]. The fusion to a carrier protein and to a synthetic propeptide, LEISSTCDA, was repeatedly shown to stabilize and improve he production and secretion in L.lactis when using the Nisin controlled expression system [33,34,35]. The LEISSTCDA effect was observed in different contexts, using different proteins and signal peptides. For example, the fusion of LEISS to Nuc-BLG resulting in the protein LEISS-Nuc- BLG led to the highest production of the hybrid protein, estimated at ~8ug/ml(~2-fold higher than Nuc-BLG).The LEISSTCDA effect was observed in different contexts, using different proteins and signal peptides.
Translocation machinery /protein targeting systems
There are tools developed for protein delivery systems in LAB for which the protein of interest is targeted to a defined cell location, i.e., the cytoplasm, the cell wall, or the medium. The expression and export vectors based on (i)Sec-dependent machinery for protein translocation across the membrane and (ii)Sortase-dependent machinery for protein anchorage to the cell wall. Studies with reporter proteins in gram-positive bacteria have shown that secretion efficiency depends on both the SP and the secretion target [36,37]. Bacteria possess several mechanisms for secretion of proteins, of which the secdependent system is most commonly explored in genetic engineering.
The Sec machinery: This is a ubiquitous secretion system comprised of a set of proteins that mediate translocation of a precursor protein (the mature protein plus an N-terminal signal peptide) across the cytoplasmic membrane [38].Sec translocation machinery in L.lactis translocon is composed of Sec A,the ATPase dependent motor,which partically provides the energy required for preprotein translocation, and SecY, Sec E and SecG integral membrane proteins ,which form the conducting channel through the hydrophobic membrane environment [39]. To date, the SP of the major lactococcal secreted protein Usp45 is one of the most widely exploited SP in genetically engineered LAB [40,41]. And there has been numerous heterologous proteins have been secreted in extracellular medium, which has been a boom for industrial production and research.
Sortase-dependent machinery: Two primary cell wallanchored proteins, a cell wall proteinase and a clumping factor, were characterized in the model LAB, L.lactis [42,43]. All the cell wall-anchored (CWA) proteins share a rather similar C-terminal anchoring tail of about 35 amino acids. The anchoring structure includes an LPXTG motif followed by a stretch of about 23 hydrophobic amino acids and 6 to 7 mostly positively charged residues at the extreme C terminus [44]. Recent studies on cell wall anchoring in Staphylococcus aureus suggest that the initial step involves export of the surface protein precursors across the membrane by a Sec-dependent mechanism and that once translocated, the hydrophobic domain and the positively charged C terminus anchor the protein through interactions with the membrane and the negatively charged phospholipids on the cytoplasmic face, respectively. A putative sortase enzyme(s) cleaves at the threonine and creates an amide linkage with the free amino group of the peptide cross-bridge in the cell wall [45,46]. Cell wall anchoring of heterologous proteins using the CWA of proteins A and M6 from S. aureus and Streptococcus pyogenes, respectively, was demonstrated with various gram-positive hosts, including LAB [47,48,49].
Roll of HtrA mutant Lactobacillus strain
HtrA is a single surface protease which is responsible for the housekeeping of exported proteins in L.lactis and other gram positive organisms. HtrA is responsible for clearing abnormal proteins, such as reporter fusions, from the surface, and is both essential and induced under several stress conditions. All tested proteins are entirely stable in htrA mutant, suggesting that this mutant is protease-free at the cell surface [50,51], a major advantage for developing L. lactis as a cell factory for protein production and secretion.
Theraupautic approaches using Lactococcus lactis
In L.lactis heterologous proteins have been directed to the cytoplasm, the cell wall, or the medium. Making use of this strategy, L.lactis have been used widely in therapeutic field which is used is different sites.
1. Production and targeting of the brucella abortus antigen L7/L12 in L.lactis: food grade live vaccine against bucellosis: targeted to cytoplasm, cell wall and extra cellular medium [52].
2. Oral vaccination of mice against rodent malaria with recombinant L.lactis expressing MSP-119[53].
3. Expression of Helicobacter pylori urease subunit B gene in L.lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice [54].
4. High potential of interleukin-12-secreting lactococci strains for future prophylactic and therapeutic uses [55].
5. Live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papilloma-virus type 16- induced tumors [56].
6. Mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papilloma-virus type 16-induced tumors [57]. 7. Genetically modified L.lactis secreting interleukin- 10 provides therapeutic approach for inflammatory bowel disease [58]
Closing remarks
In conclusion, we assume that a complete tool box is now available for heterologus protein production and targeting in L.lactis. In addition, these tools may be implemented in other Gram-Positive and even Gram-negative bacteria. The GRAS (generally regarded as safe) status of L.lactis and LAB in general, is a clear advantage for their use in production and secretion of therapeutic or vaccinal proteins.
Acknowledgements
This work was supported by a grant from the Next- Generation BioGreen 21 Program, Rural Development Administration of the Korean Ministry of Food, Agriculture, Forestry, and Fisheries (PJ0079732011).
References
- Alexandrescu AT, Hinck AP, Markley JL. Coupling between local structure and global stability of a protein: mutants of staphylococcal nuclease. Biochemistry 1990; 29: 4516-4452
- Bredmose L, Madsen SM, Vrang A, et al. Development of a heterologous gene expression system for use in Lactococcus lactis. In: Merten O-W et al (eds) Recombinant protein production with prokaryotic and eukaryotic cells. A comparative view on host physiology. Kluwer Academic Press, Dordrecht, pp 269-275
- Mierau I and Kleerebezem M, 10 years of the nisincontrolled expression system (NICE) in Lactococcus lactis, Appl Microbiol Biotechnol 2005; 68:705-717.
- Bolotin A, Wincker P, Mauger S, et al. The complete genome sequence of the lactic acid bacterium Lactococcus lactis. Genome Res.2001; 11: 731-751.
- Le Loir Y, Azevedo V, Oliveira SC, et al. Protein secretion in Lactococcus lactis: An efficient way to increase the overall heterologous protein production. Microb Cell Fact. 2005,4 (1): 2.
- Hackett J. Use of Salmonella for heterologous gene expression and vaccine delivery systems. Curr Opin Biotechnol 1993; 4: 611-615
- Holmgren J, Czerkinsky C. Cholera as a model for research on mucosal immunity and development of oral vaccines. Curr Opin Immunol 1992; 4: 387-391
- Robinson K, Chamberlain LM, Schofield KM, et al, Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol 1997; 15: 653-657.
- Kuipers OP, de Ruyter, PG, Kleerebezem M. el al. Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol 1997; 15: 135-140.
- de Vos, W.M. (1999). Gene expression systems for lactic acid bacteria. Curr. Opin. Microbiol. 2: 289-295.
- van der Vossen JM, van der Lelie D, Venema G: Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol 1987; 53: 2452-2457.
- Wells JM, Wilson PW, Norton PM, et al. Lactococcus lactis: high level expression of tetanus toxin fragment C and protection against lethalchallenge. Mol Microbiol 1995, 8: 1155-1162.
- Israelsen, H, Madsen SM, Vrang A, et al. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80.Appl Environ Microbial 1995, 61: 2540-2547.
- Walker SA, Klaenhammer TR. Molecular characterization of phage inducible middle promoter and its transcriptional activator from the lactococcal bacteriophage ?31.Journal of Bacteriology, 1998; 180: 921- 930.
- Nauta A, van den Burg B, Karsens H, et al. Design of thermolabile bacteriophage repressor mutants by comparative molecular modeling. Nat Biotechnol 1997, 15: 980-983.
- Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok J:A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbial 1998, 27. 299-310.
- Madsen SM, Arnau J, Vrang A, et al. Molecular characterization of the pH-inducible and growth phasedependent promoter P170 of Lactococcus lactis. Mol Microbiol 1999; 32: 75-87.
- de Vos WM, Kuipers OP, van der Meer JR, et al. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by gram-positive bacteria. Mol Microbiol 1995; 17: 427-437.
- Llull D, Poquet I: New expression system tightly controlled by zinc availability in Lactococcus lactis. Appl Environ Microbiol 2004; 70: 5398-5406.uenade
- von Heijne G. The signal peptide. J Membr Biol 1990; 115: 195-201.
- Pugsley, AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev 1993; 57: 50- 108.
- Nielsen H, Engelbrecht J, Brunak S, et al. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int J Neural Syst. 1997; 8: 581-599.
- von Heijne G. Patterns of amino acids near signalsequence cleavage sites. Eur J Biochem 1983; 133: 17- 21.
- Le Loir Y, Gruss A, Ehrlich S.D, et al. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol 1998; 180: 1895-1903.
- Arnau J, Madsen SM, Vrang A, et al. Optimization of signal peptide SP310 for heterologous protein production in Lactococcus lactis. Microbiology 2003; 149: 2193-2201.
- Bermudez-Humaran LG, Langella P, Cortes-Perez NG, et al. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect Immun 2003b; 71: 1887-1896.
- Dieye Y, Usai S, Clier F, et al. Design of a proteintargeting system for lactic acid bacteria. J Bacteriol 2001; 183: 4157-4166.
- Bermudez-Humaran LG, Langella P, Commissaire J, et al. Controlled intra- or extracellular production of staphylococcal nuclease and ovine omega interferon in Lactococcus lactis . FEMS Microbiol Lett 2003a; 224: 307-313.
- Mierau I, Olieman K, Mond Jet al. Optimization of the Lactococcus lactis nisin-controlled gene expression system NICE for industrial applications. Microb Cell Fact 2005b; 4: 16.
- Le Loir Y, Azevedo V, Oliveira SC,et al. Protein secretion in Lactococcus lactis : an efficient way to increase the overall heterologous protein production. Microb Cell Fact 2005; 4: 2
- Novotny R, Scheberl A, Giry-Laterriere M, et al. Gene cloning, functional expression and secretion of the Slayer protein SgsE from Geobacillus stearothermophilus NRS 2004/3a in Lactococcus lactis . FEMS Microbiol Lett 2005; 242: 27-35.
- Le Loir Y, Nouaille S, Commissaire J, et al. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis . Appl Environ Microbiol 2001; 67: 4119-4127.
- de Ruyter PG, Kuipers OP, de Vos WM. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Applied and Environmental Microbiology 1996; 62: 3662-3667.
- Kuipers OP, de Ruyter PG, Kleerebezem M et al. Quorum sensing-controlled gene expression in lactic acid bacteria. Journal of Biotechnology 1998; 64: 15- 21.
- Nouaille S, et al. Improvement of bovine ß-lactoglobulin production and secretion by Lactococcus lactis. Braz J Med Biol Res. 2005, 38; 353-359.
- Slos P, Dutot P, Reymund J, et al. Production of cholera toxin B subunit in Lactobacillus. FEMS Microbiol Lett 1998; 169: 29-36.
- Brockmeier U, Caspers M, Freudl R, et al. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J Mol Biol 2006; 362: 393-402.
- Simonen M, Palva I. Protein secretion in Bacillu species. Microbiol Rev. 1993; 57:109-113.
- Bolhuis A, Broekhuizen CP, Sorokin A, et al. SecDF of Bacillus subtilis , a molecular Siamese twin required for the efficient secretion of proteins. J Biol Chem 1998; 273: 21217–21224.
- Poquet I, Ehrlich SD, Gruss A. An export-specific reporter designed for gram-positive bacteria: application to Lactococcus lactis. J Bacteriol 1998; 180: 1904- 1912.
- Jeong DW, Lee JH, Kim KH, et al. A food-grade expression ⁄ secretion vector for Lactococcus lactis that uses an alpha-galactosidase gene as a selection marker. Food Microbiol. 2006; 23: 468-475.
- Godon JJ, Kury K, Shearman CA, et al. The Lactococcus lactis sex-factor aggregation gene cluA. Mol Microbiol 1994; 12:655-1663.
- Kok J, Leenhouts KJ, Haandrikman AJ, et al. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol 1998; 54: 231-238.
- Fischetti VA, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from Grampositive cocci. Mol. Microbiol 1990; 4:1603-1605.
- Navarre WW, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in Gram-positive bacteria. Mol. Microbiol. 1994; 14: 115-121.
- Schneewind O,Fowler A, Faull KF. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science.1995; 268: 103-106.
- Piard JC, Hautefort I, Fischetti VA. The M6 protein from Streptococcus pyogenes: cloning and expression of its emm6 structural gene and cell wall anchoring efficiency in various lactic acid bacteria. J. Bacteriol. 1997; 179: 3068-3072.
- Piard JC, Jimenez-Diaz R, Fischetti VA, et al. The M6 protein of Streptococcus pyogenes and its potential as a tool to anchor biologically active molecules at the surface of lactic acid bacteria. Adv. Exp. Med. Biol 1997; 418: 545--550.
- Sta¨hl S, M. Uhle´n. Bacterial surface display: trends and progress. Trends Biotechnol.1997; 15: 185-192.
- Poquet I, Saint V, Seznec E, et al. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol Microbiol 2000; 35: 1042-1051.
- Foucaud-Scheunemann C, Poquet I: HtrA is a key factor in the response to specific stress conditions in Lactococcus lactis. FEMS Microbiol Lett 2003; 224: 53-59.
- Luciana AR, Vasco A, Le Loir Y, et al.Applied and Environmental Microbiology 2002; 68: 910-916 52. Zhang ZH, Jiang PH, Li NJ, et al. Oral vaccination of mice against rodent malaria with recombinant Lactococ-cus lactis expressing MSP-119. World J Gastroenterol 2005; 11 (44): 6975-6980.
- Lee MH, Roussel Y, Wilks M. et al. Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice. Vaccine 2001; 16: 19 (28-29): 3927-3935.
- Bermúdez-Humarán LG, Cortes-Perez NG, Ah-Leung S, et al. Current prophylactic and therapeutic uses of a recombinant Lactococcus lactis strain secreting biologically active interleukin-12. J Mol Microbiol Biotechnol 2008; 14 (1-3): 80-89.
- Poquet I, Saint V, Seznec Eet al. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol Microbiol 2000;35: 1042-1051.
- Bermúdez-Humarán LG. Cortes-Perez NG, Lefèvre F.et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol. 2005(Dec)1;175 (11): 7297-7302.
- Lothar S, Sabine N, Nathalie H. et al. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nature Biotechnology 2003; 21: 785-789.