Case Report - Journal of Clinical Ophthalmology (2020) Volume 4, Issue 2
Retinal Complications in Donnai-Barrow syndrome: A case report.
Ali M Al-Hallafi*, Ahsan A ChaudhryDepartment of Ophthalmology, Security Forces Hospital Program, Riyadh, Saudi Arabia
- Corresponding Author:
- Dr. Ali M Al-Hallafi
Department of Ophthalmology
Security Forces Hospital Program
Riyadh
Saudi Arabia
E-mail: amm-ry@hotmail.com
Accepted date: June 08, 2020
Abstract
Donnai-Barrow syndrome (DBS) is an autosomal recessive disorder, is characterized by particular facial features, congenital diaphragmatic hernia, developmental delay, sensorineural hearing loss, rhegmatogenous retinal detachment, epilepsy and intellectual disability. As such requires early and lifelong medical surveillance.
Keywords
Donnai-Barrow syndrome, Ocular complications, retinal detachment
Introduction
Donnai-Barrow syndrome (DBS) is an inherited disease that involves many areas of the body. DBS has a characteristic craniofacial finding (large anterior fontanelle, wide metopic suture, widow's peak, markedly widely spaced eyes, enlarged globes, downslanted palpebral fissures, posteriorly rotated ears, depressed nasal bridge, and short nose. Ocular complications mainly affect posterior segments such as high progressive myopia, retinal detachment, retinal dystrophy, and gradual decreased vision. Agenesis of the corpus callosum, loss of hearing, intellectual disability, and hernia of diaphragm and omphalocele are other features [1,2]. DBS cases in general suffer from mild to moderate brain disability and developmental delay [3].
The diagnosis of DBS is established with the characteristic clinical features, distinctive pattern of low-molecular-weight proteinuria and biallelic pathogenic variants in LRP2 identified by molecular genetic testing [4].
Management includes surgical repair of diaphragmatic hernia and/or omphalocele, eye spectacles for correction of myopia, preventive treatments for retinal detachment such retinal laser for retinal breaks, hearing aids and/or cochlear implants for hearing loss, antiepileptic drugs for seizures, vitamins A and D supplements and education tailored to degree of intellectual, visual, and hearing abilities.
Surveillance includes serial ophthalmologic and audio logic examinations; serial measurements blood urea nitrogen, creatinine concentrations, vitamin A and D.
DBS is known to present genetically in an autosomal recessive (AR) performance. When parents are carriers of a pathogenic variant, each child of an affected person has a 25% chance of being affected, and a 25% chance of being normal and a 50% chance of being an asymptomatic carrier [5].
Case Presentation
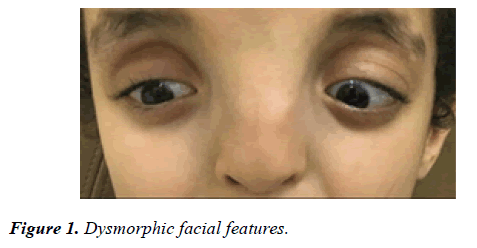
Our patient is a ten year old boy offspring of completely healthy parents. He was delivered at full term gestational period and was appropriate for the gestational age. On neonatal examination he was found to have dysmorphic facial features as shown in Figure 1.
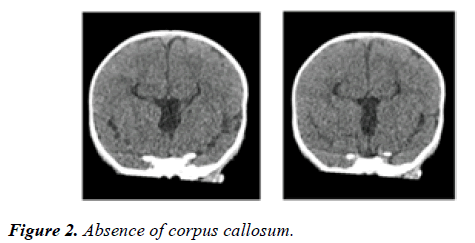
The ultrasound examination of the head showed absence of corpus callosum. Chest x-ray revealed mesocardia and elevated right hemi diaphragm.
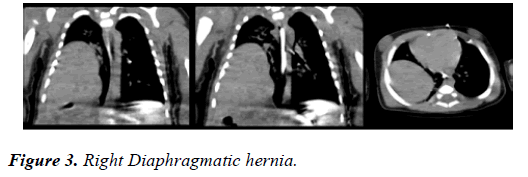
CT scan of head confirmed absence of corpus callosum, parallel lateral ventricles, continuation of inter-hemispheric fissure, high riding third ventricle and mild prominence of occipital horns of lateral ventricle [Figure 2]. The CT scan of chest and abdomen revealed right diaphragmatic hernia with large part of right love of lived in the posterior part of the chest [Figure 3].
Chest x-ray fluoroscopy showed no evidence of paradoxical or limitation of both hemi diaphragm. CT angiography of chest showed normal anatomy of chest vasculature. The surgical repair of diaphragmatic hernia was not done because of stable clinical course. He has moderate intellectual disability and developmental delay.
At the age of seven years he presented to ophthalmology services with right esotropia. On examination under general anesthesia he was found to have right chronic inoperable retinal detachment. The examination of left eye showed high myopia with normal myopic fundus. The objective refraction was -11.00 D. He was advised to have regular ophthalmology surveillance. He developed retinal detachment in left at the age of nine years and presented to emergency department with loss of vision in left eye. The examination showed hand motion vision (HM) in the left, normal anterior segment and rhegmatogenous retinal detachment. He underwent left pars plan vitrectomy, 360 degree endolaser and silicone oil temponade under general anesthesia. Although he was found to have total retinal detachment, as of this writing retina is flat.
Discussion
Even Though prevalence of DBS is unknown, it seems to be a rare entity. A few cases of DBS have been reported worldwide.
LRP2 gene mutations cause DBS. The LRP2 gene supports commands for producing a Megalin which is a receptor protein [6], this protein comes up with spots into which specific new proteins, called ligands, suited. Collectively, ligands and their receptors generate signals that disturb cell growth and performance. Megalin is implanted in the membrane of cells that line up the shells and fissures of the body. Megalin proteins and many ligands are participated in several body developments, including the absorption of vitamins A and D, immune system response stress control, the transportation of fats in the blood circulation, the growth of several body organs such as brain, spinal cord, eyes, ears, lungs, intestine, reproductive system, and renal tubules [7]. The characteristics of DBS are most likely triggered by the failure of Megalin proteins to improve absorb ligands, disturbance of biochemical signaling paths [4].
DBS is inherited in an AR form, which requires both copies of the gene in each cell have mutations. In nearly all briefcases, the parents of an individual with an AR disorder are healthy, and they are asymptomatic [8].
One case was discovered to have DBS as a consequence of uniparental disomy from his father uniparental disomy arises when diagnosis is suggested by a combination of clinical and neuroimaging features along with a typical pattern of lowmolecular- weight proteinuria, increased urinary levels of retinol-binding protein (RBP) and RBP/creatinine ratio. The diagnosis is confirmed by molecular genetic testing [4,8].
Detection of hypertelorism, agenesis of the corpus callosum, and either Congenital diaphragmatic hernia or omphalocele by prenatal imaging should raise suspicion of DBS. Prenatal diagnosis for at-risk pregnancies is possible and requires earlier recognition of the disease-triggering mutation in the family [9].
DBS is AR syndrome. Genetic counseling should be provided to parents of affected children and to their relatives. Parents of an involved newborn are required to be carriers for the diseaseproducing allele [9].
DBS has a feature group of clinical characteristics restricting differential diagnoses. Nevertheless, some corresponding signs are observed in, Chudley-McCullough, tetrasomy 12p, Fryns, Acrocallosal, and Craniofrontonasal syndromes. The renal phenotype comparatively is similar to Lowe syndrome and Dent disease. The eye phenotype could be like Stickler syndrome [10].
Routine examination of the eye to check visual acuity and fundus exam is crucial, in addition to hearing testing and kidney function. Eye spectacles, prophylactic retinal laser treatment for retinal breaks or retinal surgery for retinal detachment may be required. Adapted education for sight, hearing and intellectual incapacities has to be offered as is required for concerned kids.
Affected individuals can achieve useful vision and hearing with correction. Overall health status in patients is generally good in childhood and adolescence. End-stage kidney failure is a rare and life-threatening complication. Pre- or peri-natal presentation with diaphragmatic and abdominal wall defects requires surgical intervention and is associated with elevated morbidity and mortality.
Our patient was managed. The retinal detachment required pars plans vitrectomy and silicone oil temponade to save the remaining eye. Our massage is to keep a routine retinal exam for those patients to avoid the occurrence of major complications and keep a regular eye glasses correction, in addition to pediatric and ear consultations.
References
- Patel N, Hejkal T, Katz A, et al. Ocular manifestations of Donnai-Barrow syndrome. J Child Neurol. 2007;22:462-4.
- Pober BR, Longoni M, Noonan KM. A review of Donnai-Barrow and facio-oculo-acoustico-renal (DB/FOAR) syndrome: clinical features and differential diagnosis. Birth Defects Res A Clin Mol Teratol. 2009;85:76-81.
- Roane H, Bouxsein K, Fulton C. Assessment and treatment of self-injurious behavior associated with DonnaiBarrow syndrome. J Dev Phys Disabil. 2012;24:327-35.
- Storm T, Heegaard S, Christensen EI, et al. Megalin deficiency causes high myopia, retinal pigment epithelium-macromelanosomes and abnormal development of the ciliary body in mice. Cell Tissue Res. 2014;358:99-107.
- Donnai-Barrow Syndrome. Mauro Longoni et al In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle. 2008;1993-2020.
- Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002 ;3:256-66.
- Christensen EI, Nielsen R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2007;158:1-22.
- Kantarci S, Ragge NK, Thomas NS, et al. Donnai-Barrow syndrome (DBS/FOAR) in a child with a homozygous LRP2 mutation due tocomplete chromosome 2 paternal isodisomy. Am J Med Genet A. 2008;146A:1842–7.
- Vasudevan C, McKechnie L, Levene M. Long-termoutcome of antenatally diagnosed agenesis of corpus callosum and cerebellar malformations. Semin Fetal Neonatal Med. 2012;17:295-300.
- Donnai-Barrow syndrome. Genetic Home Reference, 2013.