Research Article - Current Pediatric Research (2023) Volume 27, Issue 5
Results of improving sleep of infants after MDO to treat severe Pierre robin sequence in Vietnam.
Dang Hoang Thom1,3*, Vu Ngoc Lam2 and Tran Thiet Son3
1Department of Pediatrics, Vietnam National Children’s Hospital, Hanoi, Vietnam
2Department of Nursing, 108 Military Central Hospitals, Hanoi, Vietnam
3Department of Plastic Reconstructive Aesthetic Surgery, Hanoi Medical University, Hanoi, Vietnam
- Corresponding Author:
- Dang Hoang Thom
Department of Plastic Reconstructive
Aesthetic Surgery, Hanoi Medical University
Hanoi, Vietnam
E-mail: drthomdh@gmail.com
Received: 26 April, 2023, Manuscript No. AAJCP-23-97378; Editor assigned: 28 April, 2023, Pre QC No. AAJCP-23-97378(PQ); Reviewed: 09 May, 2023, QC No. AAJCP-23-97378; Revised: 18 May, 2023, Manuscript No. AAJCP-23-97378(R); Published: 29 May, 2023, DOI:10.35841/0971-9032.27.05.1875-1878.
Abstract
Pierre Robin Sequence (PRS) refers to a group of birth defects that typically include micrognathia and glossoptosis. These conditions can lead to airway obstruction at the base of the tongue and may be accompanied by cleft palate. Hypoplasia of the mandible leads to glossoptosis, which results in obstruction of the upper airway, feeding difficulties and sleep-disordered breathing. But there are no studies that have reported on the complete range of sleep-related outcomes in infants with Pierre Robin sequence that had undergone Mandibular Distraction Osteogenesis (MDO).
Objective: 73 infants aged 1-12 months with a diagnosis of Pierre Robin Sequence (PRS), who had undergone MDO (MDO) at the Vietnam national children's hospital between 2019 and 2021. Methods: A longitudinal study.
Results: The 73 infants included, 32 (43.8%) were male, 11 (15.1%) were premature and 56 (76.7%) had isolated PRS. The average age at MDO was 35 days (IQR: 22 to 60 days). The infants were extubated on postoperative day 4 (IQR: 3 to 4 days). Tube feeding rate is 89.0%. The mean AHI of the subjects group decreased markedly after MDO treatment; dropped from 25.5 pre-operatively to 1.7 post-operatively (p<0.001). Obstructive Apnea Hypopnea Index (AHI) decreased from 22.1 before MDO to 1.1 after MDO. The lowest SpO2 nadir increased from 74.3% to 84.2%, whereas obstructive apneas reduced from 76.2 to 5.8, hypopneas decreased from 48.1 to 22.1 (p=0.032), and time spent below 90% SpO2 decreased from 4.3% to 0.7% (p<0.001).
Conclusion: MDO improved several architectural parameters which strongly affect to sleep with PRS.
Keywords
Sleep, Pierre robin, Jawbone, Infants
Introduction
PRS is a group of birth defects including small jaw and tongue, with or without cleft palate; can lead to airway obstruction at the base of the tongue. In the United States, the incidence of PRS is quite low, ranging from 1 in 5000 to 1 in 7000 births [1-3].
Hypoplasia of the mandible leads to glossoptosis, which can lead to feeding difficulties, Sleep-Disordered Breathing (SDB), and obstruction of the upper airway [4-6]. SDB, especially in the form of Obstructive Sleep Apnea (OSA), is an almost universal finding in infants with PRS, with prevalence ranging from 85% to 100% [7]. The morbidity associated with OSA in infants is well described and this can result in failure to thrive, developmental and learning delays, cor pulmonale, and ultimately, death [8]. Costa, et al. found in a large retrospective review that mortality at PRS was 16.7% [9].
Recent publications advocate routine Polysomnography (PSG) screening in all patients with PRS who have difficulty eating and in patients with desaturations in the prone position [10,11]. The objective nature of PSG makes it an ideal tool to guide airway management and prevent Obstructive Sleep Apnoea (OSA) related morbidity [12,13].
The treatment of airway obstruction by Pierre Robin series has a wide range of options, from observation or prone position for mild cases to different types of surgery (including tongue-lip adhesions, osteogenesis) (mandible or tracheostomy) for severe cases [14,15]. MDO has become a popular treatment for airway obstruction in infants with Pierre Robin sequence that have micrognathia, as it directly addresses the underlying issue-the hypoplastic mandible [16]. Although there has been some limited evidence for resolution of obstructive sleep apnea following MDO. However, no study has reported comprehensive sleep-related outcomes in infants with PRS after MDO [17-19]. For that reason, our aim is to describe changes in sleep-related respiratory parameters in infants with Pierre Robin sequence that underwent MDO in Vietnam.
Methodology
We performed a longitudinal study of 143 infants aged 1-12 months diagnosed with PRS who underwent MDO at the Vietnam national children's hospital in the period of 2019 to 2021. The research was approved by Hanoi medical university review board.
We did not include children older than 12 months, lacking information in the medical records. The information on each subject was reviewed simultaneously by two independent Otolaryngologists for the results unifying. We conduct physical examination of all newborns; perform sleep endoscopy, direct laryngoscopy, rigid tube bronchoscopy and PSG.
The sleep endoscopy is the use of a flexible fiberoptic endoscope to examine the airway, tongue position, and jaw thrust when the infants were supine and supine. All patients were treated by the same team of surgeons. This team will be in charge of plastic and reconstructive surgery with mandibular distraction. Postoperative PSG was performed at the end of the distraction process but before hardware removal.
General information on sex, age, and clinical symptoms related to upper airway obstruction were examined before and after MDO. FFL findings were documented in detail with drooping of the tongue in various positions and findings of laryngeal palsy. Classification pediatric otolaryngologists will classify laryngomalacia according to the mild, moderate and severe grades associated with epiglottitis collapse. During the physical examination at the time of Flexible Fiberoptic Laryngoscopy (FFL) and direct laryngoscopy and bronchoscopy, the additional signs of reflux as well as airway compromise were also noted. Regarding the method of data processing, we employ a paired sample t-test that was used to compare the pre- and post-MDO means of measurement before and after the MDO.
Results
In a cohort of 143 infants with PRS, 109 (76.2%) treated by airway-related surgery (either MDO or tracheostomy).
Seventy-third infants (66.9% ) were eventually included in the study because they met all inclusion criteria There were 36 infants who were not eligible to participate in the study due to: criteria for exclusion included being intubated at birth during polysomnography, undergoing polysomnography more than 3 months after MDO, and having undergone oxygen titration or a split-night study with oxygen. Of the 73 infants included, 32 (43.8%) were male, 11 (15.1%) were premature and 56 (76.7%) had isolated Pierre Robin sequence (Table 1).
| Demographic and clinical characteristics | All infants (%) |
|---|---|
| No. | 73 |
| Male | 32 (43.8) |
| Prematurity | 11 (15.1) |
| Isolated PRS | 56 (76.7) |
| Age at MDO, day | |
| Median | 35 |
| IQR | 22-60 |
| Post-operative intubation, days | |
| Median | 4 |
| IQR | 3-4 |
| Total hospital length of stay, days | |
| Median | 29 |
| IQR | 23-39 |
| Tube feeding (NG or GT) at pre-MDO | 65 (89.0) |
| Note: NG: Nasogastric Tube; GT: Gastrostomy Tube; IQR: Interquartile Range | |
Table 1. Demographic and clinical characteristics of infants.
The average age of MDO patients was 35 days (interquartile range, 22 to 60 days). The median age at MDO was not significantly different between infants with isolated Pierre Robin Sequence and those with syndromic Pierre Robin Sequence (36 days (interquartile range, 22 to 60) vs. 29 days (interquartile range, 21 to 85 days)).
No postoperative complications occurred and the children were extubated on postoperative day 4 (interquartile range, 3 to 4 days). The length of hospital stay was 29 days (interquartile range, 23 to 39 days). Tube feeding rate is 89.0%.
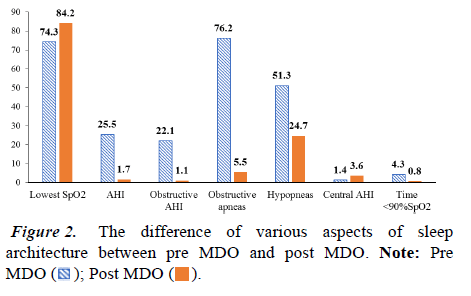
Some aspects of sleep architecture were discovered to improve following surgery. The individuals' mean AHI fell significantly after MDO treatment. The mean AHI fell from 25.5 pre MDO to 1.7 post MDO, a mean difference of 23.8 (p<0.001); obstructive AHI decreased from 22.1 before MDO to 1.1 after MDO, both of which were substantial improvements.
The lowest SpO2 nadir increased from 74.3% to 84.2%, whereas obstructive apneas reduced from 76.2 to 5.8, hypopneas decreased from 48.1 to 22.1 (p=0.032), and time spent below 90% SpO2 decreased from 4.3% to 0.7% (p<0.001). No infants have required tracheostomy as part of their airway treatment after surgery (Figures 1 and 2).
Discussion
MDO was first applied for infant’s treatment with hemifacial microsomia and treacher-collins syndrome in the 1990’s [20-22]. MDO is a less invasive option to procedures that have a higher morbidity and complication rate. Then, plenty reports have been published which demonstrating the procedure's efficiency and safety [23-25]. According to the literature, the MDO reduces the severity of OSA by preventing the need for a tracheostomy, assisting with decannulation, and reducing the need for tracheostomy [26-28].
The MDO expands the infant airway's anterior-posterior dimensions by lengthening the mandible and shifting the geniohyoid muscle attachment anteriorly. Lam, et al. demonstrated in a retrospective cohort study that MDO can prevent tracheostomy in PRS patients and facilitate decannulation in previously tracheostomised patients [29]. The overall success rate in this study was 75.6%. Meanwhile, the lower complication rate, only 26.8%, was critically higher in the group of tracheostomy than in the first MDO subgroup. Breik, et al. performed a 2016 meta-study from 66 articles that showed the success rate (defined as preventing tracheostomy) of 95.5% [26].
Among the patients in this study, the mean AHI significantly decreased from 25.5 preoperatively to 1.7 postoperatively, with a mean difference of 23.8 (p<0.001). The mean Obstructive Apnea-Hypopnea Index (OAHI) decreased from 22.1 to 1.1 after surgery. The mean lowest O2 saturation nadir increased from 74.3% to 84.2%. The SpO2 dropped from 4.3% to 0.8%. These results agree with the data from earlier sources. In the study of P. Papoff, et al. the author evaluated the treatment results of 24 PSG patients before and after MDO surgery. The study results showed that the average AHI of this group of subjects decreased from 47 to 10.9 after 1 month and further decreased to only 2.5 after 1 year. The mean lowest value of O2 saturation increased from 76.5% to 98.3% after 1 month and continued to increase to 98.5% after 1 year from the time of surgery.
This study also compared these results with a control group undergoing TLA and presented that MDO was dominant to TLA in patients without the syndrome [30]. Briek, et al. in their study found 11 articles comparing obstructive AHI before and after surgery. As a result, AHI was significantly reduced from 31.2 (before surgery) to 4.34 (after surgery), with a mean difference of 26.9 [26]. Another large retrospective study of 121 patients reported significantly reduced mean AHI across all weight groups. However, children weighing<4 kg had better reductions than children weighing>4 kg (41.5 vs. 26.1).
The study concluded that MDO is an effective treatment method for low birth weight infants with high safety and low complications. To further elucidate the relationship between sleep indices and clinical symptoms of neonatal disease, Daniel, et al. performed a recent review of the literature on neonatal PSG [31]. The author studied 10 papers on the values of various parameters of PSG in infants. The results show that the upper limit of normality for OAHI and MAI is reported to be less than 1.0. In contrast, there was a gradual decrease with age in the central apnea index. The upper limit was 45 for 1 month old infants, 30 for 2 month olds, 22 for 3 month olds, and 10-20 for elder age groups. The change in central apnea in the growing infant is thought to be due to the variation in respiratory physiology from the fetal to the infant’s period.
Conclusion
In our research sample of infants with PRS, MDO demonstrated the achievement well increasing parameters of sleep, in particular AHI. By the study of 73 children with PRS syndrome, mandibular stretching is highly effective in improving jawbone size and increasing airway size for pediatric patients. Thereby, it results in improving respiratory congestion and reducing the difficulty of nursing. The limitation of the study is that the number of infants who completely performed PSG after 3 months of MDO was still low compared to the total number of infants treated. Interobserver and internal reliability is ensured through data collection. All steps were performed exactly as described in the methods.
Conflicts of Interest
All authors in this study have no conflicts of interest, financial or otherwise.
References
- Scott AR, Mader NS. Regional variations in the presentation and surgical management of Pierre Robin sequence. Laryngoscope 2014; 124(12): 2818–25.
- Hsieh ST, Woo AS. Pierre robin sequence. Clin Plast Surg 2019; 46(2): 249–59.
- Giudice A, Barone S, Belhous K, et al. Pierre robin sequence: A comprehensive narrative review of the literature over time. J Stomatol Oral Maxillofac Surg 2018; 119(5): 419–28.
- Daniel M, Bailey S, Walker K, et al. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhinolaryngol 2013; 77(4): 499–503.
- Anderson ICW, Sedaghat AR, McGinley BM, et al. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre robin sequence. Cleft Palate Craniofac J 2011; 48(5): 614–8.
- Gómez OJ, Barón OI, Peñarredonda ML. Pierre robin sequence: An evidence-based treatment proposal. J Craniofac Surg 2018; 29(2): 332–8.
- Cladis F, Kumar A, Grunwaldt L, et al. Pierre robin sequence: A perioperative review. Anesth Analg 2014; 119(2): 400–12.
- Singer LP, Saenger P. Complications of pediatric obstructive sleep apnea. Otolaryngol Clin North Am 1990; 23(4): 665–76.
- Costa MA, Tu MM, Murage KP, et al. Robin sequence: Mortality, causes of death, and clinical outcomes. Plast Reconstr Surg 2014; 134(4): 738–45.
- Reddy VS. Evaluation of upper airway obstruction in infants with Pierre robin sequence and the role of polysomnography–Review of current evidence. Paediatr Respir Rev 2016; 17: 80–7.
- Zaballa K, Singh J, Waters K. The management of upper airway obstruction in Pierre robin sequence. Paediatr Respir Rev 2022; S1526-0542(22): 00047-1.
- Dennison WM. The Pierre robin syndrome. Pediatrics 1965; 36(3): 336–41.
- Insalaco LF, Scott AR. Peripartum management of neonatal Pierre robin sequence. Clin Perinatol 2018; 45(4): 717–35.
- Perrino MA. Neonatal mandibular distraction. Atlas Oral Maxillofac Surg Clin North Am 2022; 30(1): 57–62.
- Rossini G, Vinci B, Rizzo R, et al. Mandibular distraction osteogenesis: A systematic review of stability and the effects on hard and soft tissues. Int J Oral Maxillofac Surg 2016; 45(11): 1438–44.
- Hong P, Bezuhly M. Mandibular distraction osteogenesis in the micrognathic neonate: A review for neonatologists and pediatricians. Pediatr Neonatol 2013; 54(3): 153–60.
- Côté A, Fanous A, Almajed A, et al. Pierre robin sequence: Review of diagnostic and treatment challenges. Int J Pediatr Otorhinolaryngol 2015; 79(4): 451–64.
- Lee JC, Bradley JP. Surgical considerations in Pierre robin sequence. Clin Plast Surg 2014; 41(2): 211–7.
- Meshram GG, Kaur N, Hura KS. Pierre robin sequence: Diagnostic difficulties faced while differentiating isolated and syndromic forms. Acta Medica (Hradec Kralove). 2020; 63(2): 86–90.
- McCarthy JG. The role of distraction osteogenesis in the reconstruction of the mandible in unilateral craniofacial microsomia. Clin Plast Surg 1994; 21(4): 625–31.
- Moore MH, Guzman-Stein G, Proudman TW, et al. Mandibular lengthening by distraction for airway obstruction in treacher-collins syndrome. J Craniofac Surg 1994; 5(1): 22–5.
- Aljerian A, Gilardino MS. Treacher collins syndrome. Clin Plast Surg 2019; 46(2): 197–205.
- Earley M, Butts SC. Update on mandibular distraction osteogenesis. Curr Opin Otolaryngol Head Neck Surg 2014; 22(4): 276–83.
- Hatefi S, Hatefi K, Le Roux F, et al. Review of automatic continuous distraction osteogenesis devices for mandibular reconstruction applications. Biomed Eng Online 2020; 19(1): 17.
- Starch-Jensen T, Kjellerup AD, Blæhr TL. Mandibular midline distraction osteogenesis with a bone-borne, tooth-borne or hybrid distraction appliance: A systematic review. J Oral Maxillofac Res 2018; 9(3): e1.
- Breik O, Tivey D, Umapathysivam K, et al. Mandibular distraction osteogenesis for the management of upper airway obstruction in children with micrognathia: A systematic review. Int J Oral Maxillofac Surg 2016; 45(6): 769–82.
- Niño-Sandoval TC, Rodrigues EDR, Vasconcelos BC. Latency phase in mandibular distraction osteogenesis: A systematic review in animal models. Br J Oral Maxillofac Surg 2021; 59(9): 993–1004.
- Shakir S, Bartlett SP. Modern mandibular distraction applications in hemifacial microsomia. Clin Plast Surg 2021; 48(3): 375–89.
- Lam DJ, Tabangin ME, Shikary TA, et al. Outcomes of mandibular distraction osteogenesis in the treatment of severe micrognathia. JAMA Otolaryngol Neck Surg 2014; 140(4): 338–45.
- Papoff P, Guelfi G, Cicchetti R, et al. Outcomes after tongue–lip adhesion or mandibular distraction osteogenesis in infants with Pierre robin sequence and severe airway obstruction. Int J Oral Maxillofac Surg 2013; 42(11): 1418–23.
- Daniel KN, Chan CH. A review of normal values of infant sleep polysomnography. Pediatr Neonatol 2013; 54(2): 82–7.

 ).
).