Research Article - Biomedical Research (2017) Volume 28, Issue 20
Research on the mechanisms of zerumbone in inhibition of proliferation and induction of apoptosis in human esophageal cancer cell line Eca-109
Changjiang Lei1,2#, Manli Yan2#, Bin Wang2#, Hong Ren1*, Yuan Li2, Qingyun Pan21Department of Surgical Oncology, the First Affiliated Hospital of Xi'an Jiaotong University, 710061, PR China
2Department of Oncology, the Fifth Hospital of Wuhan, 430050, PR China
#Changjiang Lei and Manli Yan and Bin Wang contributed equally to this work.
- *Corresponding Author:
- Hong Ren
Department of Surgical Oncology
Beijing Tongren Hospital
The First Affliated Hospital of Xi’an Jiaotong University, PR China
Accepted date: September 26, 2017
Abstract
Objective: To investigate the mechanisms of zerumbone in proliferation and inducing effect on apoptosis of human esophageal cancer cell line Eca-109.
Methods: Eca-109 cell lines were divided into two zerumbone treatment groups (10 μmol/L and 20 μmol/L) and one blank control group (0 μmol/L). Thereafter, MTT assay was performed to observe the inhibitory effect of zerumbone on the in-vitro growth of Eca-109 cells; flow cytometry was performed for detecting the distribution of cell cycles; inverted phase contrast microscope was used to observe the morphology of Eca-109 cells; RT-PCR and Western blotting assay were carried out for detecting the changes in mRNA and protein expressions of CXCR4.
Results: Zerumbone had a significant inhibitory effect on the growth of Eca-109 cells, and the inhibitory effect was continuously elevated with an increase in concentration (p<0.05). Besides, zerumbone could induce the apoptosis of Eca-109 cells, and the apoptotic rate was increased in a concentration-dependent manner (p<0.05). After 48 h and 72 h of treatment with zerumbone in different concentrations, we found that the incidence of poor adhesive growth and cell death was increased against the augmentation in concentration of zerumbone; with the time extension, the quantity of Eca-109 cells would be decreased gradually after treatment with zerumbone in a certain concentration. The results of RT-PCR and Western blotting assay also showed that compared with the control group, the mRNA and protein expressions of CXCR4 in 10 μmol/L and 20 μmol/L groups were significantly decreased (p<0.05), in which the decrease in 20 μmol/L group was more significant.
Conclusion: Zerumbone can inhibit the growth of Eca-109 cells with a promoting effect on apoptosis, and the mechanism might be correlated with the downregulation of CXCR4.
Keywords
Zerumbone, Human esophageal cancer, Eca-109, CXCR4.
Introduction
Zerumbone, a kind of sesquiterpenes extracted from herbal, is characterized with the anti-inflammatory effect and anti-tumor effect in some studies [1]. Some other studies [2] showed that zerumbone can inhibit the invasion of breast cancer cells mediated by CXCR4. Currently, there remain few studies reporting the inhibitory and inducing effects of zerumbone on proliferation and apoptosis of Eca-109 cells, respectively, and the intrinsic mechanism of zerumbone in inducing cell apoptosis is still unclear. CXCR4, a highly conservative Gprotein- coupled receptor, has been confirmed to be involved in a variety of biological behaviors in many tumors, such as cell proliferation, metastasis, invasion and apoptosis [3,4]. In this study, human esophageal cancer Eca-109 cell lines were treated with zerumbone in different concentrations, thereby investigating the mechanism of zerumbone in inhibiting the proliferation and inducing the apoptosis in human esophageal cancer cells, and its effect on the mRNA and protein expressions of CXCR4.
Materials and Methods
Cell culture
Eca-109 cells (Institute of Biochemistry and Cell Biology, SIBS, CAS) in logarithmic phase were enrolled in the control group and combined group, and cultured in the RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 0.1% penicillin and streptomycin. Cell culture was carried out in presence of HEPES/3% glutamine (pH 7.2-7.2) at 37°C and 5% CO2 in an incubator.
Detecting the growth inhibition ratio
Eca-109 cells were inoculated onto a 96-well plate at density of 1 × 104/ml and cultured at 37°C for 24 h in an incubator containing 5% CO2. Zerumbone in different concentrations (10 μmol/L and 20 μmol/L) was used for treatment of cells for 48 h and 72 h, while the cells in control group received the treatment of zerumbone in concentration of 0 μmol/L, in which 6 replicative wells were set in each group. In each well, 10 μL MTT reagent (5 mg/mL) was dropped followed by 4 h of culture at 37°C and 5% CO2. Afterwards, 100 μL DMSO was added into each well followed by vibration for 10 min and detecting the optical density (OD) at wavelength of 570 nm to calculate the growth inhibition rate of Eca-109 cells according to the following formula: growth inhibition rate (%)=(1-OD drug treatment group/OD control group) × 100% [5].
Observing the cell morphology under the inverted phase-contrast microscope
Eca-109 cells were inoculated onto a 96-well plate at density of 1 × 104/mL. After 48 h and 72 h, cells were placed under the inverted phase-contrast microscope (200X) to observe the morphological changes in cells in blank control group and the drug-treatment groups.
Analysis of changes in cell cycleCells in logarithmic phase were collected and inoculated into the culture flask at density of 1 × 104/mL (10 mL/flask, 6 flasks for each group). After culture at 37°C overnight, 10 and 20 μmol/L zerumbone was respectively added into the drugtreatment groups, while the DMEM medium in same volume was added into the control group. Culture was terminated at 12 h. After 24 h, centrifugation was carried out to collect the Eca-109 cells in control group and drug-treatment groups. These cells were rinsed with phosphate-buffer saline (PBS) three times, and stained with propidium iodide in a dark place for detection via FCM.
Detecting the mRNA expression of CXCR4 via RTPCR
After 48 h of treatment, RNA extraction kit (TaKaRa) was applied to extract the total RNA from Eca-109 cells in each group for reverse transcription, in which the primers were designed with Primer Premier 5.0 and synthesized by Sangon Biotech (Shanghai) Co., Ltd. CXCR4 primer sequences are shown as follows: forward 5'- AAAATCTTCCTGCCCACCAT-3', reverse 5'- TTGCTATCGTCTGCCTCACT-3', 440 bp. GAPDH primer sequences are shown as follows: forward 5'- AGAAGGCTGGGGCTCATTTG-3', reverse: 5'- AGGGGCCATCCACAGTCTTC-3', 258 bp. PCR was carried out in following conditions: initial denaturation at 94°C for 5 min; 30 cycles of 94°C for 30 s, annealing at 55°C (for CXCR4) and 59°C (for GAPDH) for 30 s and extension at 72°C for 1 min; extension at 72°C for 5 min. Products were separated via electrophoresis on 2% agarose, and the electrophoresis results were collected by gel imaging system for analysis of gray value with Quantity One and calculation of the ratios of CXCR4 band to the GAPDH band. This experiment was conducted in triplicate.
Detecting the protein expression of CXCR4 via Western blotting assay
After 48 h of treatment, RIPA agent was added into the cells for lysis to extract the total protein. Following the quantification analysis of CXCR4 with BCA kit, we took 50 μg protein for electrophoresis on SDS-PAGE, and then the protein was transferred onto the PVDF membrane. After the membrane was blocked with skimmed milk, the primary antibodies of CXCR4 (1:300) and GAPDH (1:500) were added onto the membrane for incubation at 4°C overnight followed by rinsing with TBST. Then, HRP-labeled goat anti-rabbit IgG (1:1000) was added for incubation for 1 h. Thereafter, color development was performed for proteins with ECL kit followed by gray value analysis in Quantity One, and this experiment was conducted in triplicate.
Statistical analysis
SPSS 21.0 was utilized to perform one-way ANOVA, and SNK test was adopted for analysis of the significant differences between groups, in which p<0.05 suggested that the difference had statistical significance.
Results
Results of MTT assay
Zerumbone exhibited significant inhibitory effect on the growth of Eca-109 cells, and the inhibitory effect was continuously elevated with an increase in concentration (p<0.05, Table 1).
| Groups | Inhibition Rate (%) | |
|---|---|---|
| 48 h | 72 h | |
| Control | 0 | 0 |
| 10 µmol/L Zerumbone | 15.76 ± 1.38 | 29.95 ± 3.15 |
| 20 µmol/L Zerumbone | 27.05 ± 3.12 | 49.37 ± 4.03 |
| F | 11.031 | 17.654 |
| p | <0.05 | <0.05 |
Table 1: The MTT assay results of several groups (n=6).
Variations in distribution of cell cycle in each group
Besides, zerumbone could induce the apoptosis of Eca-109 cells, and the apoptotic rate was increased in a concentrationdependent manner (p<0.05; Table 2).
| Groups | Cell cycle | Apoptotic cells | ||
|---|---|---|---|---|
| G0-G1 | S | G2-M | ||
| Control | 53.16 ± 2.48 | 34.03 ± 3.05 | 9.16 ± 2.76 | - |
| 10 µmol/L zerumbone | 17.05 ± 0.32 | 8.75 ± 0.52 | 63.43 ± 0.47 | 3.48 ± 0.12 |
| 20 µmol/L zerumbone | 22.72 ± 1.22 | 24.58 ± 2.01 | 47.31 ± 1.05 | 10.22 ± 1.05 |
| F | 21.054 | 19.823 | 16.741 | 22.006 |
| p | <0.05 | <0.05 | <0.05 | <0.05 |
Table 2: Distribution of cell cycle in each group (n=6).
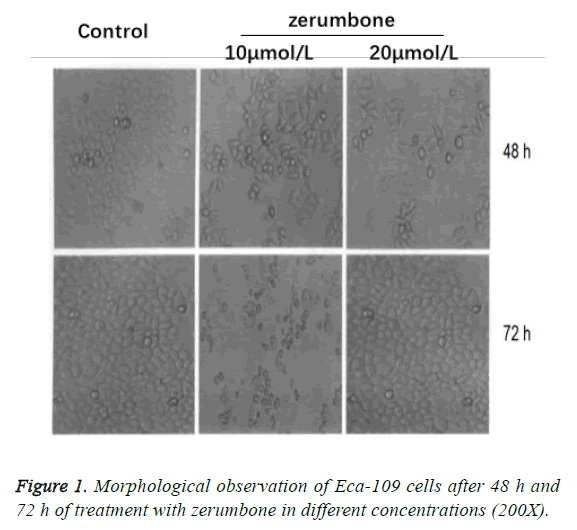
Morphological observation of Eca-109 cells
Cells in the control group grew well and presented in intensive adhesive growth pattern. After 48 h and 72 h of treatment with zerumbone in different concentrations, we found that the incidence of poor adhesive growth and cell death were increased against the augmentation in concentration of zerumbone; with the time extension, the quantity of Eca-109 cells would be decreased gradually after treatment with zerumbone in a certain concentration (Figure 1).
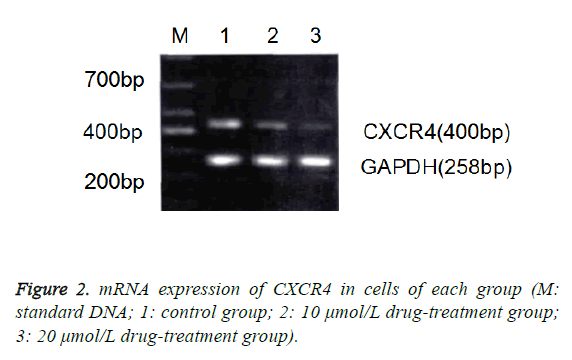
Effect of zerumbone on gene expression of CXCR4 in Eca-109 cells
The results of RT-PCR and Western blotting assay also showed that compared with the control group, the mRNA and protein expressions of CXCR4 in 10 μmol/L and 20 μmol/L groups were significantly decreased (p<0.05), in which the decrease in 20 μmol/L group was more significant (Figure 2).
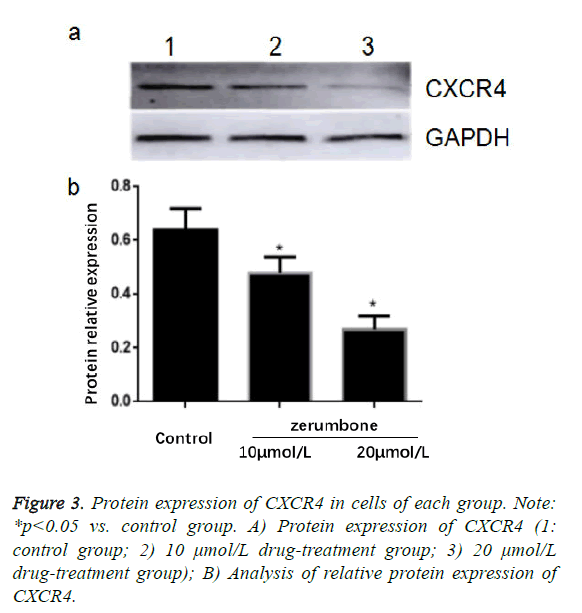
Effect of zerumbone on protein expression of CXCR4 in Eca-109 cells
The results of Western blotting assay showed that compared with the control group, the protein expressions of CXCR4 in 10 μmol/L and 20 μmol/L groups were significantly decreased (p<0.05), in which the decrease in 20 μmol/L group was more significant (Figure 3).
Discussion
Literatures [6,7] have indicated that zerumbone, a cytotoxic ingredient extracted from the root and stem of wild ginger, can significantly inhibit cell growth with the potential anti-tumor effect. Some other studies [8,9] also revealed that zerumbone is strongly cytotoxic to lymphocytes. Currently, inducing the apoptosis of tumor cells but without any effect on surrounding normal cells and tissues has been regarded as a key indicator in evaluating the efficacy of chemotherapeutics. The results of this study showed that after treatment with zerumbone for Eca-109 cells, the MTT assay showed that the growth of Eca-109 cells was significantly inhibited in a dosagedependent manner, suggesting that zerumbone can suppress the growth of Eca-109 cells.
Activated CXCR4 will initiate the transduction of ERK/MAPK signal pathways, thereby upregulating the relative genes [10,11]. Existing studies [12,13] indicated that CXCR4 are expressed in tumor tissues like breast cancer and ovarian cancer. Some studies [14,15] reported that after the expression of CXCR4 was silenced by RNAi, the proliferation rate of breast cancer cell would be decreased, and the suppression on activity of CXCR4 could promote cell apoptosis. The results of this study showed that in Eca-109 cells that were treated with zerumbone, there were decreases in mRNA and protein expressions of CXCR4, and with an increase in concentration, the decreases would be more evident, suggesting that zerumbone can inhibit the growth of Eca-109 cells and promote the apoptosis, which might be associated with the downregulation of CXCR4.
In conclusion, we found that after Eca-109 cells were treated with zerumbone, the growth of esophageal cancer cells could be inhibited; besides, zerumbone could significantly inhibit the growth of Eca-109 cells and promote the apoptosis possibly through suppressing the mRNA and protein expressions of CXCR4. However, in-depth research is required to further elucidate the mechanism of zerumbone inhibiting the growth and promoting the apoptosis of cancer cells.
Acknowledgement
This work was supported by Natural science foundation of Hubei province (2016CFB591)
References
- Arigami T, Natsugoe S, Uenosono Y. CCR7 and CXCR4 expression predicts lymph node status including micrometastasis in gastric cancer. Int J Oncol 2009; 35: 19-24.
- Duan C, Chen K, Yang G. HIF-1α regulates Cx40-dependent vasodilatation following hemorrhagic shock in rats. Am J Transl Res 2017; 9: 1277-1286.
- Zhang M, Zhang S, Hui Q. β-Hydroxybutyrate facilitates fatty acids synthesis mediated by sterol regulatory element-binding protein1 in bovine mammary epithelial cells. Cell Physiol Biochem 2015; 37: 2115-2124.
- Tsuboi K, Kodera Y, Nakanishi H. Expression of CXCL12 and CXCR4 in pT3-stage gastric cancer does not correlate with peritoneal metastasis. Oncol Rep 2008; 20: 1117-1123.
- Duan C, Li T, Liu L. Efficacy of limited fluid resuscitation in patients with hemorrhagic shock: a meta-analysis. Int J Clin Exp Med 2015; 8: 11645-11656.
- Wang L, Zhang J, Cai L. Liposomal curcumin inhibits Lewis lung cancer growth primarily through inhibition of angiogenesis. Oncol Lett 2012; 4: 107-112.
- Lin SS, Lai KS. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett 2009; 285: 127-133.
- Zhelev Z, Ivanova D, Bakalova R. Synergistic cytotoxicity of melatonin and new-generation anticancer drugs against leukemia lymphocytes but not normal lymphocytes. Anticancer Res 2017; 37: 149-159.
- Su CC, Wang MJ, Chiu TL. The anti-cancer efficacy of curcumin scrutinized through core signaling pathways in glioblastoma. Int J Mol Med 2010; 26: 217-224.
- Singh AP, Arora S, Bhardwaj A. CXCL12/CXCR4 signaling axis induces SHH expression in pancreatic cancer cells via ERK- and Akt- mediated activation of NF-κB: implications for bidirectional tumor-stromal interactions. J Biol Chem 2012; 55: 31-33.
- Tsuboi K, Kodera Y, Nakanishi H. Expression of CXCL12 and CXCR4 in pT3-stage gastric cancer does not correlate with peritoneal metastasis. Oncol Rep 2008; 20: 1117-1123.
- Ryang DY, Joo YE, Chung KM. Expression of c-FLIP in Gastric Cancer and its Relation to Tumor Cell Proliferation and Apoptosis. Korean J Int Med 2007; 22: 263-269.
- Nowakmarkwitz E, Puła B, Szajnik M. Expression of survivin, SDF-1 and CXCR4 on tumor cells in ovarian cancer. Ginekologia Polska 2010; 81: 674-677.
- Hou K, Lin C, Chen C. Association of probiotics and bone mineral density in Chinese patients with type 2 diabetes. Biomed Res India 2017; 28: 129-133.
- Yang P, Liang SX, Huang WH. Aberrant expression of CXCR4 significantly contributes to metastasis and predicts poor clinical outcome in breast cancer. Curr Mol Med 2014; 14: 174-184.