Review Article - Journal of Biochemistry and Biotechnology (2017) Industrial Biotechnology
Release of alkali metals during biomass thermal conversion.
Wenhan Cao1, Jun Li1*, Leo Lue1, Xiaolei Zhang2
1Department of Chemical and Process Engineering, James Weir Building, 75 Montrose Street, University of Strathclyde, Glasgow G1 1XJ, UK
2School of Mechanical and Aerospace Engineering, Queen’s University Belfast, Belfast, BT9 5AH, UK
- Corresponding Author:
- Dr. Jun Li
Department of Chemical and Process Engineering
University of Strathclyde
United Kingdom
E-mail: jun.li@strath.ac.uk
Accepted Date: November 29, 2016
Citation: Wenhan Cao, Jun Li, Leo Lue, et al. Release of alkali metals during biomass thermal conversion. Arch Ind Biotechnol. 2016; 1(1): 1-3
Abstract
Biomass has great potential to become an economic source of renewable energy; however, its high chlorine and alkali metal content may cause series problems (e.g. slagging and corrosion) thus limiting its utilization. This paper reviews the release of potassium during biomass thermal conversion. Organic potassium is released first when the temperature is relatively low, starting at about 473 K and slowing down at about 773 K; the release of inorganic potassium occurs with the increase of processing temperature. The potassium vapors are mainly in the form of KCl, KOH and K2SO4. In addition to the temperature, the properties of biomass, fuel-air ratio, pressure and heating rate also significantly influence the release rate of alkali metals. Future studies are required to develop accurate kinetic models of potassium release to address the ash related challenges when firing and co-firing biomass with high inherent alkali content.
Keywords
Biomass, Alkali metal, Release mechanism.
Introduction
Biomass is material derived from living or recently living organisms and is a potential source of energy with very specific properties. Biomass has great potential to replace fossil fuels in energy systems, offering environmental benefits due to its carbon neutrality and renewability. However, the firing and co-firing of biomass can lead to problems such as slagging, fouling and metal corrosion, due to its high chlorine, potassium and sodium content. During thermochemical conversion, the alkali species can be released from biomass through vaporization; the gaseous species can react further or undergo physical transformations to either condense on fly ash or form aerosols, which can further deposit on heat transfer surfaces and lead to complex metal reactions [1] resulting in corrosion on these surfaces due to the interaction between the alkali species and metals.
Ash related slagging and fouling remains a major technical challenge in the thermochemical conversion of biomass. Better understanding of the release behavior of alkali metal species is crucial to overcome these problems. Consequently, there have been several studies to determine the yield and release rate of alkali metals, especially potassium [2-5]. Generally, potassium is an essential macro-nutrient for growing plants, especially woody plants, while sodium is not an essential stimulant and actually toxic at higher concentrations. In fact, the proportion of potassium is approximately one to two orders-of-magnitude larger than that of sodium in wood [6,7]. This has also been reflected by the size of the literature, as studies of the release and influence of potassium are more common than those of sodium [8]. In consequence, this paper considers potassium as representative alkali metals and its release behavior during the biomass thermal conversions will be reviewed.
Release of Alkali Metals
Alkali metals in biomass mainly exist in three forms: organic form (e.g. R-COO-K+), inorganic salts (e.g. KCl, K2SO4) and mineral form (e.g. K2O3Si). Minor amounts of bounded potassium are considered stable and nonvolatile during thermal conversion, so the study of release of potassium focuses on the organic and inorganic forms. During devolatilization, organic alkali metals and loosely bonded free ions are primarily released at relatively low temperatures, while the release of more strongly bonded alkali metals requires a higher temperature that normally occurs at the char oxidation stage [1]. The occurrence of chemical reactions and phase transformations of alkali metals in the different stages depends on the temperatures of the gases and solids and the presence of other compounds in the ash, such as chlorine and sulfur. Analysis of these aspects of gas-phase potassium release during biomass combustion has been presented in recent years [9-14].
Experiments carried out in Ref [15] showed that only a small amount of potassium is released, mainly in elemental form at the early stage of devolatilization. During devolatilization, potassium is mainly released as KCl, while the amount of KOH released is relatively small. The release of KOH increases at the char burnout stage. KCl, K2SO4 and KOH are the common potassium species released from biomass during thermochemical conversation [16]; also, the potassium most likely exists in biomass as a dissolved salt in the moisture or as a cation bound to a functional group, e.g. –COOH [17], and the released potassium is likely to form KCl that highly depends on the chlorine content.
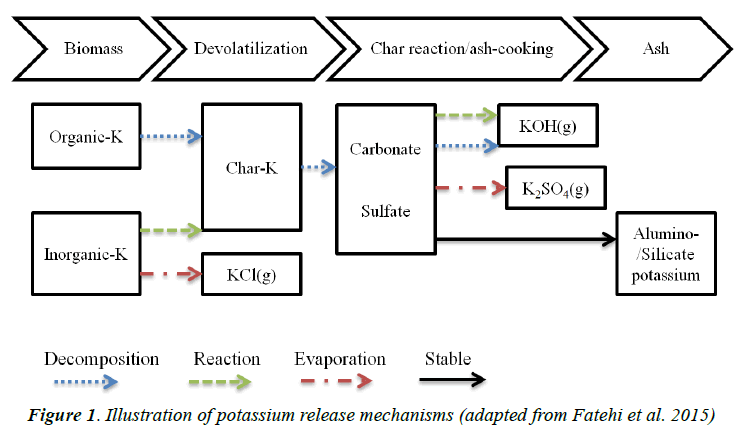
Biomass combustion can be described as an initial pyrolysis (to produce gaseous, liquid products and char) followed by char oxidation. Pyrolysis dictates the quantity and type of alkali metals to be distributed into solid, liquid and gas phases. The release of potassium into the vapor phase during biomass pyrolysis happens in two stages [18]. The first stage occurs at temperatures in range of 473 K to 773 K, during which the release of potassium accompanies the evaporation of volatiles during biomass pyrolysis and is independent of the chlorine content of biomass. Organic potassium in biomass is released at low temperatures [16], which occur at the first stage of pyrolysis. When the temperature of pyrolysis is higher than 773 K, at the second stage, the potassium release is highly dependent on the chlorine content, and the inorganic potassium (KCl and KOH) is vaporized into a vapor phase, as illustrated in Figure 1.
Figure 1 depicts a reaction mechanism for the release of potassium from biomass with low chlorine content [18]. The mechanism reveals, at the pyrolysis stage, that inorganic potassium is partly released into the gas phase in the form of KCl, while the rest of the inorganic potassium and organic potassium remains in the char residue (Char-K). Following the ash-cooking stage, the char reacts with CO2/O2/ H2O to form carbonate, sulfate and silicate groups. The reaction of carbonate with O2/H2O produces gaseous potassium in the form of KOH, which is dependent on the partial pressure of water vapor in the surrounding gas; the evaporation of potassium sulfate can lead to the formation of K2SO4, and the remaining potassium stay in the ash in alumino-/silicate form.
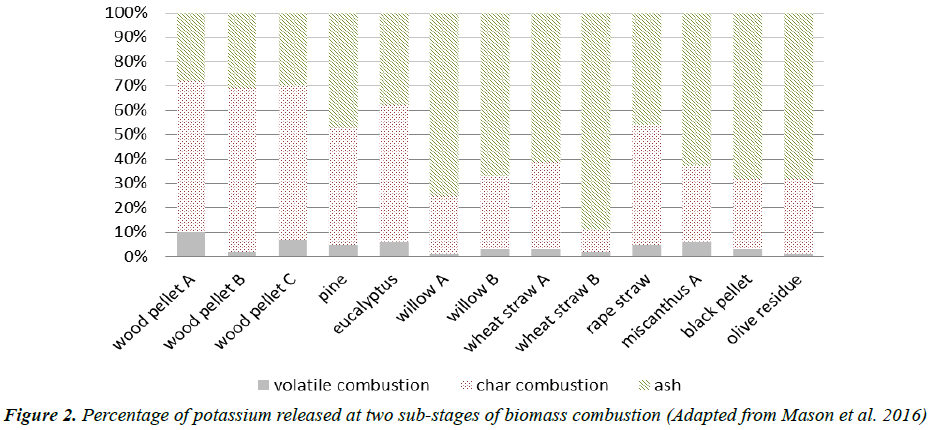
Although a clear demarcation between devolatilization and char combustion is still challenge, it has been reported that the duration of devolatilization can be estimated by single particle experiments [19]. Accordingly, the relative proportion of potassium released at the devolatilization and char combustion stages could be determined. Figure 2 shows that the fraction of potassium released during each stage varies with sample. Notably, the major part of potassium of most woody biomass is released at the char combustion stage, however, the potassium of most herbaceous biomass is more stable and mainly remains in the condensed-phase ash.
Using a lab scale combustion simulator for different types of biomass, the investigation of [20] found that potassium release in the gas phase is significant; however, the correlation with its original concentration in biomass is not linear, and the presence of other inorganic species (e.g. silicon, aluminum, calcium, and magnesium) have potential impact on the gas phase releases of potassium and sodium. The aluminum and silicon content in biomass act as a buffer [21], which makes the alkali metals significantly less volatile during thermal conversions. In addition, the density, shape and particle size of biomass may also influence significantly the contribution of the inorganic releases during thermochemical conversions [20,22].
Conclusion
The alkali metals in biomass exist in organic, inorganic and mineral forms. As the temperature is increased, the organic alkali metals start to release. When the temperature is high enough, inorganic alkali metals start to release. The alkali metals released in gaseous phase are mainly in forms of KOH, KCl and K2SO4. The release of alkali metals occurs through complex reactions, which are influenced by many factors: the content of other inorganic species; density, shape, and particle size of the biomass; fuel to air ratio; heating rate and temperature, etc. Future fundamental studies on the release kinetics of alkali metals are required to determine the yield of released alkali metals, their form, and the release rate. These results will help to significantly advance ash deposition models and furnace design, thus, promoting the future exploitation of biomass as a sustainable resource for energy production.
References
- Haykiri-Acma H, Yaman S. Interaction between biomass and different rank coals during co-pyrolysis. Renew Energ 2010;35:288-292.
- Akbar S, Schnell U, Scheffknecht G. Modeling potassium release and the effect of potassium chloride on deposition mechanisms for coal and biomass-fired boilers. Combust TheorModel 2010;14:315-329.
- Fatehi H, He Y, Wang Z, et al. LIBS measurements and numerical studies of potassium release during biomass gasification. Proc Combust Inst 2015; 35:2389-2396.
- Mason PE, Darvell LI, Jones JM, et al. Observations on the release of gas-phase potassium during the combustion of single particles of biomass.Fuel 2016; 182:110-117.
- Sorvajarvi T, DeMartini N, Rossi J, et al. In situ measurement technique for simultaneous detection of K, KCl, and KOH vapors released during combustion of solid biomass fuels in a single particle reactor. ApplSpectrosc 2014; 68:179-184.
- Van LithSP, Jensen F, Frandsen P, et al. Release to the gas phase of inorganic elements during wood combustion. Patr2: Influence of fuel composition. Energy Fuels 2008; 22:1598-1609.
- Werkelin J, Lindberg D, Bostrom D, et al. Ash-forming elements in four Scandinavian wood species part3: Combustion of five spruce samples. Biomass Bioenerg2011; 35:725-733.
- Saddawi A, Jones JM, Williams A. Influence of alkali metals on the kinetics of the thermal decomposition of biomass. Fuel Process Technol 2012; 104:189-197.
- Blomberg T. A thermodynamic study of the gaseous potassium chemistry in the convection sections of biomass fired boilers. Mater Corros 2011; 62:635-641.
- Li R, Kai X, Ynag T, et al. Release and transformation of alkali metals during co-combustion of coal and sulfur-rich wheat straw. Energy Convers Manag 2014; 83:197-202.
- FrandsenFj, Van LithSC, Korbee R, et al. Quantification of the release of inorganic elements from biofuels. Fuel Process Technol 2007; 88:1118-1128.
- Kim SS, Kang YS, Lee HD, et al. Release of potassium and sodium species during combustion of various rank coals, biomass, sludge and peats. J IndEngChem 2012; 18:2199-2203.
- Sorvajarvi T, DeMartini N, Rossi J, et al. In situ measurement technique for simultaneous detection of K, KCl, and KOH vapors released during combustion of solid biomass fuels in a single particle reactor. ApplSpectrosc 2014; 68:179-184.
- Westberg HM, Bystrom M, Leckner B. Distribution of potassium, chlorine, and sulfur between solid and vapor phases during combustion of wood chips and coal. Energy Fuels 2003; 17:18-28.
- Sommersacher P, Kienzl N, Brunner T, et al. Simultaneous online determination of S, Cl, K, Na, Zn and Pb release from a single particle during biomass combustion. Part 1: experimental setup-implementation and evaluation. Energy Fuels 2015; 29:6734-6746.
- Bjorkman E, Stromberg B. Release of chlorine from biomass at pyrolysis and gasification conditions. Energy Fuels 1997; 11:1026-1032.
- Wei X, Schnell U, Hein KRG. Behaviour of gaseous chlorine and alkali metals during biomass thermal utilisation. Fuel 2005; 84:841–848.
- Yu C, Zhang W. Progress in Themochemical Biomass Conversions.Blackwell Science Ltd., Oxford UK 2008.
- Mason PE, Darvell LI, Jones JM, et al. A single particle flame-combustion studies on solid biomass fuelsl. Fuel 2015; 151:21-30.
- Shah VK, Cieplik MK, Bertrand CI, et al. Correlating the effects of ash elements and their association in the fuel matrix. Fuel Process Technol 2010; 91:531-545.
- Doshi V, VuthaluruHB, Korbee R, et al. Development of Modelling Approach to Predict Ash Formation during Co-Firing of Coal and Biomass. Fuel Process Technol 2009; 90:1148-1156.
- Johansen JM, Jakobsen JG, FrandsenFJ, et al. Release of K, Cl, and S during pyrolysis and combustion of high-chlorine biomass. Energy Fuels 2011; 25:4961-4971.