Research Article - Journal of Advanced Surgical Research (2018) Volume 2, Issue 1
Relationship of prophetic factors associated with antioxidative, immune system and micronutrients status in breast cancer patients underwent surgical procedures.
Arif Malik1*, Rabia Rasool1, Sulayman Waquar1, Mahwish Arooj2, Maira Mahmood Bukhari3, Mahmood Husain Qazi4
1Department of Molecular Biology and Biotechnology, University of Lahore, Pakistan
2Department of Biochemistry, Fatima Memorial Hospital, Lahore, Pakistan
3Department of History University College of Medicine and Dentistry, Pakistan
4Department of Biotechnology and Informatics, Pakistan
- *Corresponding Author:
- Arif Malik
Department of Molecular Biology and Biotechnology
University of Lahore
Pakistan
Tel: +92 42-7515460-7
Fax: +92-42-7515519
E-mail: arifuaf@yahoo.com
Accepted date: June 30, 2017
Abstract
Background: Surgical intervention induces additional stress also affecting the dynamics of antioxidative, micronutrients and immune systems. Surgical treatments can deepen already existing injury in the immune system of cancer patients. Objective: The aims of the present study were to appraise the prognostic factors associated with anti-oxidative, micronutrients status and immune systems in patients with breast cancer underwent surgical procedures. Methodology: Current study estimated the levels of (SOD, CAT, GPx, GRx) and non-enzymatic antioxidants (Vit-A, Vit-C, Vit-E), micronutrients (Zn, Fe, Mn, Se, Cu) along with oxidative biomarkers (MDA, IL-10) in patient?s serum samples before and after surgical intervention. The results were compared with healthy individuals via different lab tests and ELIZA kits. Results: The enzymatic antioxidants such as SOD, CAT, GSH and GPx were considerably low in females with breast cancer as compared to healthy females. Which further decreases after surgery Results before and after surgical intervention of breast cancer patients displayed that non-enzymatic antioxidant Vit-A (78.91 ? 6.5 ?g/ml vs. 59.32 ? 8.3 ?g/ml), Vit-C (1.06 ? 0.38 ?g/ ml vs. 0.99 ? 0.11 ?g/ml), and Vit-E (2.47 ? 0.79 ?g/ml vs. 2.25 ? 0.57 ?g/ml) were decreased. Similarly increasing trends within MDA (8.04 ? 1.37 nmol/ml vs. 2.57 ? 0.81nmol/ml), IL-10 (312 ? 0.71pg/ml vs. 263 ? 1.97 pg/ml) were depicted in patients and were further increased after surgical interventions. The scrutiny of micronutrients (Fe, Zn, Mn, Cu and Se) in controls and breast cancer females shows significant decline. Conclusion: The current study portrays that fluctuation from normal levels of micronutrients and antioxidants significantly contributes in the progression of breast cancer. Already existing damage in the immune system of breast cancer patients get more intensified because of surgical intervention which is responsible for increased cytokines and oxidative stress that reduces s because of inflammation that enhance interleukins and oxidative stress which is responsible for decrease in antioxidant capability.
Keywords
Breast cancer, surgery intervention, micronutrients, antioxidants, vitamins.
Introduction
Breast cancer is the most common cancer in women all over the world in milieu of prevalence and highest mortality rate [1]. It is illustrated as a multi stage process consist of a series of epigenetic and genetic changes result in un-controlled proliferation, division of cells and apoptosis of cells and DNA repair [2]. Each breast has 15 to 20 lobes along with their lobules which are connect with each other through thin ducts. The most common breast cancer type is formed in the cells of these ducts called ductal cancer. Another type that originates from lobes or lobules is known as lobular cancer [3]. Inflammation of breast tissues can cause least type of cancer known as inflammatory breast cancer [4]. Rare types of breast cancer are medullary carcinoma that forms a distinct boundary between tumour tissue and normal tissue, mucinous carcinoma formed by the mucus releasing cancerous cells and tubular carcinoma etc. Every year, 1.3 million Females are diagnosed by breast cancer contributing 1/5th of all cancer types initiated in women. According to IARC report, the highest global burden of breast cancer is found to be 55% reported in developed countries but rapidly growing rate in developing regions have given rise to concern [5]. Approximately one in nine Pakistani female is estimated to suffer from breast cancer at some age of their lives [6]. The breast cancer aetiology illustrated it as multi factorial disease. The major risk factors are age, early menarche, use of contraceptive medications, family history, delayed menopause, hormonal therapy, obesity and benign breast cancer disease history which exert their effect via oxidative stress [7-10]. Early age menarche, first birth at older age, diminished parity, breast feeding and menopause are all coupled with sequence of breast cancer risk factors [11]. The risk of developing breast cancer is increased in patients who use contraceptives and have Hormone Replacement Therapy (HRT) than women without hormonal therapy [12].
Many researches showed that the incidence of breast cancer can be diminished with giving birth in right age and breast feeding in early stages after birth [13]. More than 1/3th of all cancer cases are preventable by long term strategy for the control of cancer by the awareness of disease, accurate diet and moderate physical activity [14]. The treatment of breast cancer depends on different factors including type of cancer, size and stage at which cancer is detected and its metastasis. The prefer treatment of breast cancer is surgery in which the complete breast or some part of it is removed when it is localized with adjuvant hormonal therapy. Sometimes surgery is in combination with radiation therapy which comprises of high resolution gamma rays accurately targeting the tumour area, destroying microscopic cancer cells and medicinal treatments. Radiation therapy is given in advanced cancer stages instead systemic treatments including chemotherapy, hormonal therapy and immune therapy are given alone or in combination. A drug treatment called Neo-adjuvant chemotherapy is given before surgery for reducing the size of tumour [15]. Chemotherapy given to cancer patients varies according to the size, location, lymph node status and age of patients. After the completion of chemotherapy if tumour is still positive, hormonal therapy is given to further treat the cancer patients [16]. Damage induced by increased oxidative stress play a remarkable role in the incidence and progression of breast cancer. Excessive ROS productions, genetic variation in antioxidant enzymes capacity, oestrogen treatment are mechanisms involved in enhancing oxidative stress [9-17]. Several dietary macronutrients which have antioxidant properties including selenium, vitamin A, vitamin C, tocopherol, vitamin D have proposed to reduce the breast cancer risk [18,19]. The aim of study was to investigate total antioxidant capacity in breast cancer patients and find out the relation between enzymatic and none-enzymatic antioxidant and micronutrient in selected breast cancer population.
Aims and Objectives
Aim of the current study was to find out the relationship of different factors that were associated with the antioxidants and micronutrients status of breast cancer patients who have underwent the surgical intervention.
Materials and Methods
A total of 53 patients diagnosed with breast cancer (Stage I-II) in the Department of Surgery (Jinnah Hospital, Allama iqbal Medical College Lahore-Pakistan). All patients (45-70 years of age) underwent surgical treatment. The study was approved by the bioethics Committee, The University of Lahore, and written informed consent was obtained from all the patients. Patients were grouped by cancer stage. Three samples of blood serum of each patient were tested before the treatment and at 7 and 14 days post operatively. Twenty five (Females) apparently healthy staff of Teaching Hospital, served as controls. None of the controls was on any medication (including alcohol, cigarette and multivitamins), had history of chronic infections, malnutrition syndrome, depression, psychosis or metabolic dysfunction (such as diabetes mellitus, liver diseases, cancer) that could interfere with their oxidative metabolites and thyroid hormone status. The experimental protocol was approved by the Research Ethical Committee of The Institute of molecular biology and biotechnology, The University of Lahore. Five (5) ml of venous blood sample was taken from the anti-cubical vein of each participant. The sample bottle was centrifuged within one hour of collection, after which the serum was separated and stored at -70°C until assayed. Following parameters were investigated: MDA was evaluated calorimetrically by using the method of Ohkawa et al. [59]. Catalase levels were detected by using Aebi preparations [60], GPx is done by [61], and SOD was found out by the method of Kakkar et al. [62]. GRx was estimated by [60]. Vit-E was estimated by Rusenberg, [63], Vit-A [64] and Vit-C was detected by using the method of Lowry et al. [65]. IL-10 was carried out by their BioV endor Human ELIZA Kits. Micronutrients (Fe, Cu, Se, Zn, and Manganese were detected by atomic absorption spectrometry.
Statistical Analysis
Data was expressed as mean ± SD (standard deviation). The significance of the results was assessed by the SPSS t-test. A p-value <0.05 was considered statistically significant. One way ANOVA and spearman correlation (Two Tailed) was used to correlate the different variables. The difference was considered significant at p<0.05.
Results
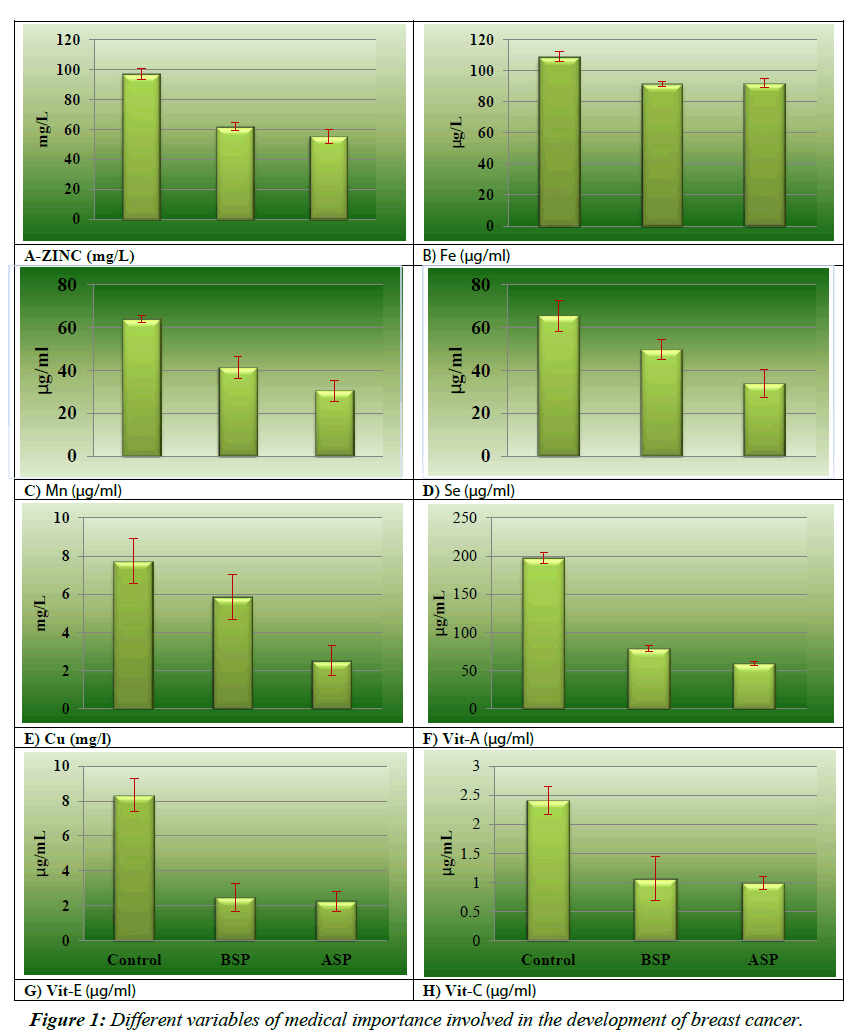
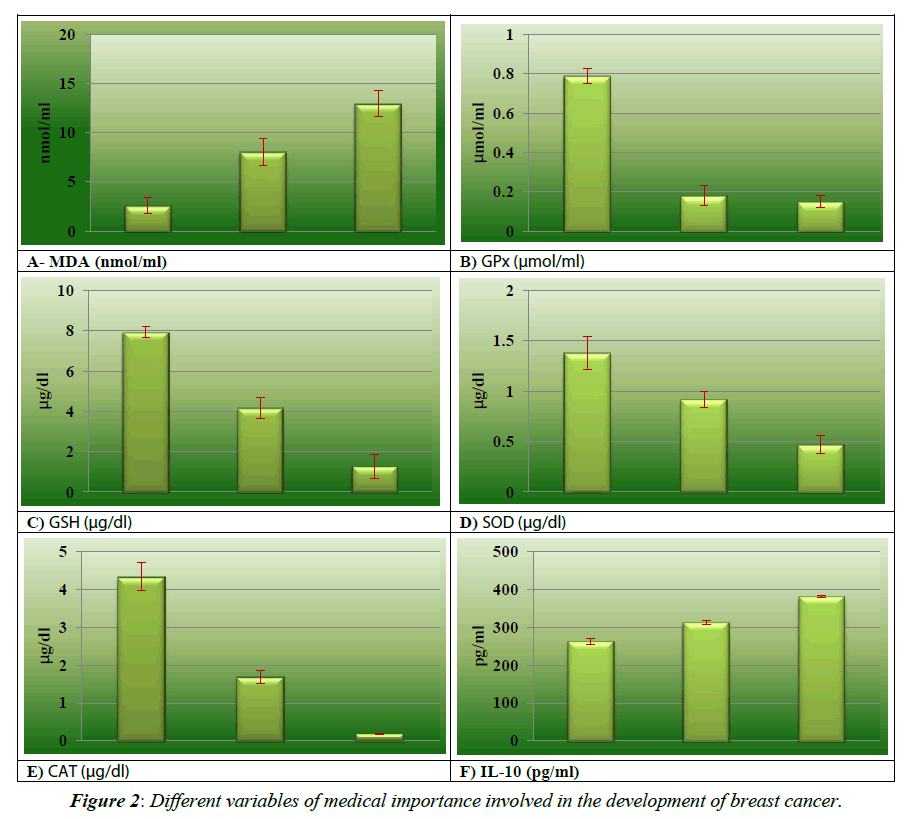
Initial comparison of enzymatic and none-enzymatic antioxidant and micronutrients of breast cancer patients during the development and after surgical procedures have been shown in the (Figures 1 and 2). The results of micronutrients showed that Zn, Se and Mn were decreased in breast cancer patients 61.67 ± 2.67 mg/L, 41.33 ± 5.23 μg/ml and 49.65 ± 4.72 μg/ml respectively as shown in the Figure 1A, 1C and 1D), as compared to the healthy controls 97.12 ± 11.76 mg/L, 65.57 ± 7.24 μg/ml, 64.05 ± 1.70 μg/ml respectively. Cu (7.73 ± 1.19 mg/L vs. 5.87 ± 1.98 mg/L) and Fe (108.95 ± 3.33 μg/ml vs. 91.33 ± 3.62 μg/ ml) results as shown in the Figure 1B and 1E) reveal drastically reduced interpretation between diseased and control groups while in pre-operative and post-operative group the levels of Zn (55.39 ± 2.71 mg/L), Cu (2.51 ± 0.23 mg/L), Mn (30.55 ± 4.88 μg/ml) and Se (33.88 ± 6.35 μg/ml) are in decreasing trend while and Fe (91.83 ± 2.94 μg/ml) are in slightly increasing pattern. Data analysis in regarding oxidative stress biomarkers MDA as shown in Figure 2A (8.04 ± 1.37 nmol/ml vs. 2.57 ± 0.81 nmol/ ml) and IL-10 as in the Figure 2F (312 ± 0.71 pg/ml vs. 263 ± 1.97 pg/ml) showed highly significant pattern between breast cancer females and controls. This trend further augmented after surgical intervention of breast cancer because of inflammatory events. As contrast with normal, considerable (p=0.045, 0.036, 0.009 and 0.047 respectively) low levels of SOD (1.38 ± 0.16 μg/dL vs. 0.92 ± 0.81 μg/dL), CAT (4.33 ± 0.74 μg/dL vs. 1.69 ± 1.18 μg /dL), GSH (7.93 ± 0.28 μg/dL vs. 4.16 ± 0.53 μg/ dL) and GPx (0.79 ± 0.34 μmol/ml vs. 0.18 ± 0.05μmol/ml) in breast cancer group as shown in Figure 2C- 2E). Results of before and after surgical intervention of breast cancer patients showed that non-enzymatic antioxidant Vit-A (78.91 ± 6.5μg/ ml vs. 59.32 ± 8.3 μg/ml), Vit-C (1.06 ± 0.38 μg/ml vs. 0.99 ± 0.11 μg/ml), and Vit-E (2.47 ± 0.79 μg/ml vs. 2.25 ± 0.57 μg/ml) as in the Figure 1F-1H showed significantly decreasing pattern and these values depicted lower levels as compared to normal females. The levels of antioxidant biomarkers including enzymatic and non-enzymatic further decreases after surgery because of increases stressed conditions.
Discussion
In present study, various antioxidants and micronutrients have been investigated in breast cancer patients preoperatively and postoperatively and also compare their levels with healthy individuals. Extreme oxidative stress can affect many cellular functions which include metabolism of cell, intracellular signaling pathways, gene regulation pathways, proliferation, and apoptosis in cell [20,21]. Damage induced by increased oxidative stress play a remarkable role in the incidence and progression of breast cancer. Excessive ROS productions, genetic variation in antioxidant enzymes capacity, oestrogen treatment are mechanisms involved in enhancing oxidative stress [9-17].This detrimental oxidative stress damage induces lipid peroxidation and increasing inflammatory events and it is also involved in the inactivation of certain tumour suppressor genes [22,23]. Certain chemotherapeutics and radio-therapeutically procedures and surgical intervention may promote the generation of ROS via oxidative stress mechanism that can further worsen the state of tumour cells result in harming healthy tissues as evident from the findings of current study. Malondialdehyde (MDA), lipid peroxidation’s secondary by-product, can boost reactive oxygen species generation in breast carcinogenesis [24]. In current study, MDA levels are elevated in preoperative and postoperative breast cancer patients than healthy individual which are similar with the findings of Rajneesh et al and Sharhan et al. reporting elevated MDA levels in breast cancer patients in comparison with controls [25,26]. The presence of cytokines in microenvironment of tumour portrays their involvement in the cancer cell growth and metastasis. IL-10 is an inflammatory biomarker secreted by activated monocytes, T cells and B cells that have dual proliferative as well as inhibitory role in the initiation and progression of breast cancer [27]. Tumourinhibitory property of IL-10 is its anti-angiogenic affect that flair its protective part against tumour. As tumour promoting role, IL-10 prevents T cells or macrophage cytokine synthesis and declines MHC class I and II antigen expression and suppress their antigen presenting capacity [28] and activates ILT4 via promoting its activity in monocytes [29,30]. IL-10 levels in present study have been shown to be increased in breast cancer patient as compared to healthy persons that confers growth and progression of tumour as reported in LIanes-Fernandez et al. [31]. Level of IL-10 is enhanced after surgery due to on-going oxidative stress and inflammation.
Many studies have reported the drastic change in antioxidant components in breast cancer women [25-34]. The present study demonstrated low levels of antioxidant in patients with breast cancer which further reduced after their surgical intervention which is concurrent with the findings of Kasapovic et al. [33]. which has reported low plasma levels of SOD, CAT, GSH, GPx and GRx in females having breast cancer. These diminished levels are because of enhanced oxidative stress in tumour cells. The levels of vitamin A and vitamin E have shown to be low in breast cancer patients supporting oxidative stress hypothesis in breast cancer. Our results are in accordance with the results of Sharhan et al [25]. Vitamin C has shown to impede the release of various inflammatory cells including CRP, IL-6, TNF-α and different types of ROS [35-37]. This study has evident diminished levels of vitamins C in breast cancer females as compared to normal individuals (Table 1). The antioxidant levels are also reduced in breast cancer patients underwent surgical procedures as reported by current study. This is due to the elevated levels of oxidative stress and inflammatory process in the area of surgical procedures.
| SUBJECTS (n=53) | |
|---|---|
| AGE < 45 Years 45-70 Years > 70 Years |
4 49 - |
| Body Mass Index Under weight Normal weight Over weight |
7 38 8 |
| Height < 160 160-170 > 170 |
2 49 2 |
| Ethnicity Caucasian African African American Others |
53 - - - |
| Smoking Non-smokers Smokers |
53 - |
| Staging Stage 0 Stage I Stage II Stage III Stage IV |
- 4 49 - - |
| Size of Tumor ≤ 2 2-5 ≥ 5 |
6 47 - |
Table 1: demographic distribution of different variables within the females of breast cancer underwent surgical intervention.
Trace elements act contribute in many biological processes occurring at cellular levels via different mechanism [38]. They at their normal range involved in maintenance of homeostasis. In breast cancer development, they might have role in oxidative DNA damage as well as immune and endocrine system. Selenium, a unique and essential trace element, evidenced to have chemo preventive and anti-cancer properties. It has a major role in activating DNA reproduction, and as a component of GPx, it protect the cellular component from the destructive effect of ROS [39]. Seleno-enzymes’ antioxidant properties are relevant to the progression of tumour as well as it activates the P53, tumour suppressor gene, that prevent proliferation process, increase DNA repair and encourages apoptosis [40]. Many studies have shown the inverse association of selenium with breast cancer [41-43] which supports the results of current study that showed their reduced levels in breast cancer as compared to normal as shown in Table 1. Fe, Cu, Mn and zinc are also some essential trace elements that form catalytic compound when combined with proteins. Zinc via its involvement in immune system as a part of T lymphocytes prevents the development of cancer [45]. Zinc increases the risk of cancer due to its role in proliferation of cell and gene transcription while it also has antioxidant ability as being the part of Cu/Zn SOD. The levels of zinc in current study is reduced in preoperative and postoperative breast cancer patients as compared to control group which is concurrent with Ding et al. [46] and Pavithra et al. [47]. The current study has reported the significant low levels of Manganese in breast cancer patients as compared to healthy individual which are in accordance with many studies [48-50]. These reduced levels of Mn are due to its anti-carcinogen properties and imbalance between antioxidant and oxidant system [51-53]. Cu and iron are involved in catalysis of many redox reactions. Fe also plays a part in cell growth regulation [44]. Copper as a part of many enzymes such as uricase, tyrosinase and cytochrome oxidase is involved in cellular metabolism and also contribute in angiogenesis as co-factor. Many studies have evident the levels of copper to be consistently high in breast cancer disease [54,55]. The findings of the present study have showed its reduced levels in breast cancer patients as compared to normal individuals which are similar to the finding of Adedapo et al. [56]. These diminished levels are due to the copper uptake by cancer cells from the blood. Iron is a essential trace element plays a major role in microenvironment of tumour. The acquisition, efflux, and regulation of iron pathway are perturbed in the presence of cancer [57]. The process of angiogenesis is also associated with iron deficiency. Hypoxia inducible factor- 1α (HIF) is hydrolyzed by HIF-1α prolyl-4-hydroxylase which has iron as an important cofactor. So, iron deficiency inhibits this hydroxylation and enhances angiogenesis [58-67]. The present study demonstrated low levels of Fe in breast cancer patients’ serum which is in line with the study of Jian et al. [57]. that evident the involvement of iron deficiency in promoting tumour development. The levels of copper and zinc in this study are reported to be further reduced after surgical interventions which are concurrent with the study of Ali et al. [66]. while the levels of Fe are slightly raised after the surgical procedure due to increased circulating oestrogen that aid the discharge of free iron from ferritin storage which in turn induce oxidative stress by catalysing the production of ROS [67]. The study was oriented for the smaller sample size due to financial constrains efficacy of the study can be increased by increasing patients to sufficient number.
Acknowledgement
The authors are highly thankful for the valuable contribution of Prof. Dr. M.H. Qazi, Director Centre for Research in Molecular Medicine (CRIMM), The University of Lahore-Pakistan regarding financial support and critical review of the manuscript.
Conflict of Interest
Authors declare no conflict of interest.
References
- Park JH, Bae SH, Jung YS, et al. Quality of life and symptom experience in breast cancer survivors after participating in a psycho educational support program: a pilot study. Cancer nursing. 2012;35: 34-41.
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends in genetics. 1993;9:138-41.
- Oliveira TM, Elias Jr J, Melo AF, et al. Evolving concepts in breast lobular neoplasia and invasive lobular carcinoma, and their impact on imaging methods. Insights into imaging. 2014;5:183-94.
- Dawood S, Cristofanilli M. Inflammatory breast cancer: what progress have we made oncology. 2011; 25:264.
- Breast cancer awareness sessions in corporate, 2011 Accessed 26 Feb 2013.
- McGuire S. World cancer report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer, WHO Press, 2015. Advances in Nutrition: An International Review Journal. 2016;7: 418-19.
- Nelson NJ. Migrant studies aid the search for factors linked to breast cancer risk. J Natl Cancer I. 2016;98: 436-8.
- Izquierdo A, Gispert R, Saladie F, et al. Analysis of cancer incidence, survival and mortality according to the main tumoral localizations, 1985-2019: Breast cancer. Medicinal clinical. 2008;131:50-2.
- Badid N, Ahmed FZ, Merzouk H, et al. Oxidant/antioxidant status, lipids and hormonal profile in overweight women with breast cancer. Pathology & Oncology Research. 2010;16:159-67.
- Gupta RK, Patel AK, Kumari R, et al. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: a case control study. Asian Pacific Journal of Cancer Prevention. 2012;13:6295-8.
- Pherson M K, Steel C, Dixon JM. Breast cancer-epidemiology, risk factors, and genetics. BMJ: British Medical Journal. 2000;21:624.
- Beral,V. Breast Cancer And Hormone Replacement Therapy In The Million Women Study. Lancet. 2003;362:419-27.
- American Cancer Society. Cancer facts and figures. 2008.
- World Health Organization (WHO). Global health risks: 5. mortality and burden of disease attributable to selected major risks. Geneva: Switzerland, WHO 2009.
- Liu SV, Melstrom L, Yao K, et al. Neoadjuvant therapy for breast cancer. Journal of surgical oncology. 2010;101:283.
- Nordgard SH. Genetic background and molecular phenotypes of breast cancer. From single gene variants to pathways and whole genome patterns. PhD thesis. The university Foundation for student life. 2008;11.
- Omar ME AS, Eman R Y, Hafez F H. The antioxidant status of the plasma in patients with breast cancer undergoing chemotherapy. Open Journal of Molecular and Integrative Physiology. 2011;1:29-35.
- Pan SY, Zhou J, Gibbons L, et al. Antioxidants and breast cancer risk-a population-based case-control study in Canada. BMC Cancer. 2011;11:372.
- Grober U, Holzhauer P, Kisters K, et al. Micronutrients in Oncological Intervention. Nutrients.2016; 8:163.
- Poli G, Leonarduzzi G, Biasi F, et al. Oxidative stress and cell signalling. Curr Med Chem. 2004;11: 1163-82.
- Kim MC, Cui FJ, Kim Y. Hydrogen peroxide promotes epithelial to mesenchymal transition and steam ness in human malignant mesothelioma cells. Asian Pac J Cancer Prev.2013;14: 3625-30.
- Hristozov D, Gadjeva V, Vlaykova T,et al. Evaluation of oxidative stress in patients with cancer. Arch Physiol Biochem. 2001;109: 331-6.
- Haris C. Individual variation among humans in carcinogen metabolism,DNA addict formation and DNA repair carcinogenesis. 1989;10:1563-6.
- Tas F, Hansel H, Belce A, et al. Oxidative stress in breast cancer. Med Oncol. 2005;22:11-15.
- Rajneesh CP, Manimaran A, Sasikala KR, Adaikappan P. Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J. 2008;49:640-3.
- Sharhan S, Normah H, Fatimah A, et al. Antioxidant intake and status, and oxidative stress in relation to breast cancer risk: A case-control study. Asian Pac J Cancer Prev. 2008;9:343-50.
- Hamidullah, Changkija B and Konwar R. Role of interleukin-10 in breast cancer. Breast cancer research and treatment. 2012;133:11-21.
- Matsuda M, Salazar F, Petersson M. Interleukin 10 pretreatment protects target cells from tumor- and allospecific cytotoxic T cells and down regulates HLA class I expression. J Exp Med.1994; 180:2371-76.
- Hashimoto SI, Komuro I, Yamada M. IL-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J Immunol. 2001;167:3619-25.
- Moore KW, Garra A, Malefyt R.Interleukin-10.Annu Rev Immunol. 1993;11:165-190.
- Llanes-Fernandez L, Alvarez-Goyanes RI, Arango-Prado Mdel C, et al. Relationship between IL-10 and tumor markers in breast cancer patients Breast. 2006;15:482-89.
- Portakal O, Ozkaya O, Erden Inal M, et al. 10 concentrations and antioxidant status in tissues of breast cancer patients. Clin Biochem. 2000;33:279-84.
- Kasapovic J, Pejic S, Todorovic A, et al. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages. Cell Biochem Funct. 2008;26:723-30.
- Yuvaraj S, Premkumar VG, Vijayasarathy K, et al. Augmented antioxidant status in tamoxifen treated postmenopausal women with breast cancer on co-administration with coenzyme Q10, niacin and riboflavin. Cancer Chemother Pharmacol. 2008;61:933-41.
- Hartel C, Strunk T, Bucsky P et al. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004; 27:101-6.
- Federico A, Morgillo F, Tuccillo C, et al. Chronic inflammation and oxidative stress in human carcinogénesis. International Journal of Cancer. 2007;121:2381-86.
- Mikirova N, Casciari J,Rogers A, et al. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. Journal of Translational Medicine. 2012;10:189-98.
- Ebrahim AM, Eltayeb M, Shaat M,et al. Study of selected trace elements in cancerous and noncancerous human breast tissues from Sudanese subjects using instrumental neutron activation analysis. Sci Total Environ. 2007;383:52-8.
- Moradi M, Eftekhari MH, Talei A, et al. A comparative study of selenium concentration and glutathione peroxidase activity in normal and breast cancer patients. Public health nutrition. 2009;12:59-63.
- Van tveer PI, Van Der Wielin RP, Kok FJ, et al. Selenium in diet, blood, and toenails in relation to breast cancer: a case-control study. American journal of epidemiology. 1990;131:987-94.
- Sunde RA .Selenium In: Bowman BA, Russell RM (editors) Present knowledge in nutrition”, 9th edn. ILSI Press, Washington, D.C. 2009:480-97.
- Suzana S. Relationship between selenium and breast cancer: a case-control study in the Klang Valley,Singap Med J. 2009;50:265-8.
- Babaknejad N, Sayehmiri F, Sayehmiri K, et al. The relationship between selenium levels and breast cancer: a systematic review and meta-analysis. Biological trace element research. 2014;159:1-7.
- Bowen H. Trace elements in biological samples. Tech Instrum Anal Chem. 1988; 8:1-17.
- Millos J, Costas-Rodriguez M, Lavilla I, et al. Multielemental determination in breast cancerous and noncancerous biopsies by inductively coupled plasma-mass spectrometry following small volume microwave-assisted digestion. Anal Chim Acta. 2008;622:77-84.
- Ding X, Jiang M, Jing H, et al. Analysis of serum levels of 15 trace elements in breast cancer patients in Shandong, China. Environmental Science and Pollution Research. 2015;22:7930-5.
- Pavithra V, Sathisha TG, Kasturi K, et al. Serum levels of metal ions in female patients with breast cancer. Journal of clinical and diagnostic research: JCDR. 2015;9:25.
- Shen F, Cai WS, Li JL, et al. The association between deficient manganese levels and breast cancer: a meta-analysis. International journal of clinical and experimental medicine. 2015;8:36-71.
- Wu HD, Chou SY, Chen DR et al. Differentiation of serum levels of trace elements in normal and malignant breast patients. Biol Trace Elem Res. 2006;113:9-18.
- Kilic E, Saraymen R, Demiroglu A, et al. Chromium and manganese levels in the scalp hair of normals and patients with breast cancer. Biol Trace Elem Res. 2004;102:19-25.
- Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk--a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. 2014;23:12-31.
- Babaknejad N, Sayehmiri F, Sayehmiri K, et al. The relationship between selenium levels and breast cancer: a systematic review and meta-analysis. Biol Trace Elem Res. 2014;159:1-7.
- Feng JF, Lu L, Zeng P, et al. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int J Clin Oncol. 2012;17:575-83.
- Shams N, Said SB, Salem TA, et al. Metal-Induced Oxidative Stress in Egyptian Women with Breast Cancer. J Clinic Toxicol. 2012;2:2161-495.
- Mahmood AA, Bilal KM Ibrahim RT. Influence of Some Trace Elements and Biochemical Parameters on Breast Cancer. Journal of Education Science. 2012;25:34-43.
- Adedapo KS, Uche CZ, Ogundiran TO. Serum trace metals in diagnosis and prognosis of post-menopausal breast cancer in a tertiary health institution in Nigeria. Archives of Applied Science Research. 2014;6:129-34.
- Jian J, Yang Q, Shao Y, et al. link between premenopausal iron deficiency and breast cancer malignancy. BMC cancer. 2013 ;13:307.
- Huang X. Does iron have a role in breast cancer, the lancet oncology. 2008;9:803-7.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95: 351-58.
- Aebi H, Bergmeyer HU: Methods in Enzymatic Analysis, Academic Press Inc, New York. 1974;3: 673-86.
- David M, Richard JS. Glutathione Reductase. Methods of enzymatic analysis. 1983;3:258-65.
- Kakkar PB, Das P, Viswanathan PN. A Modified Spectrophotometer Assay of Superoxide Dismutase. Ind J Biochem Bio. 1984; 21:130-32.
- Rosenberg IH, Miller JW. Nutritional factors in physical and cognitive functions of elderly people. The American journal of clinical nutrition. 1992;55:1237-43.
- Rutkowski M, Grzegorczyk K, Gendek E, et al. Laboratory convenient modification of Bessey method for vitamin A determination in blood plasma J. Physiol. Pharm. 2006;57:221.
- Lowry OH, Lopez JA, Bessey OA. The determination of ascorbic acid in small amounts of blood serum. J Biol Chem. 1945;162:609-15.
- Ali AA, Gurashi RA, Abdrabo AA. Assessment of Plasma Copper and Zinc Levels among Sudanese Breast Cancer Patients in Taiba Cancer Center. Journal of Medical and Biological Science Research. 2017;3:1-4.
- Galaris D, Skiada V, Barbouti A. Redox signaling and cancer: the role of “labile” iron. Cancer Lett. 2008;266:21-9.