Research Article - Biomedical Research (2017) Volume 28, Issue 10
Relationship of metformin with the risk of pancreatic cancer in patients with type 2 diabetes: a meta-analysis
Hong Hu1, Yong Fang2, Xiaoyun Zhou1, Liu Gong1, Lili Liu2, Wei Wang2, Jiping Sun1, Chongya Zhai1, Hong Pan1, Yong Dong1 and Hongming Pan2*
1Department of Medical Oncology, Xiasha Campus of Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, P.R. China
2Department of Medical Oncology, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, P.R. China
- *Corresponding Author:
- Hongming Pan
Department of Medical Oncology
Sir Run Run Shaw Hospital
Zhejiang University
PR China
Accepted date: February 25, 2017
Abstract
Aims: This study is to systematically investigate the relationship between metformin and risk of pancreatic cancer in patients with type 2 diabetes mellitus.
Methods: A systematic literature search was performed on PubMed, Embase, Cochrane Library, Highwire, CBM, CNKI, Wanfang, VIP databases. Cohort or case control studies of metformin and risk of pancreatic cancer in patients with type 2 diabetes mellitus were included. The Newcastle-Ottaua Scale score was used for quality evaluation and meta-analysis was performed using RevMan5.2 Meta-analysis software.
Results: Nine studies were included in this study. The results showed that the risk of pancreatic cancer was significantly reduced in metformin treatment group (RR=0.61, 95% CI (0.55, 0.67), P<0.001). Subgroup analysis showed that patients with type 2 diabetes in treatment with metformin and sulfonylurea (RR=0.57, 95% CI (0.51, 0.64),P<0.001) or insulin (RR=0.61, 95% CI (0.53, 0.70), P<0.001) could reduce the risk of pancreatic cancer.
Conclusions: Metformin treatment reduces the risk of pancreatic cancer in patients with type 2 diabetes.
Keywords
Pancreatic cancer, Metformin, Diabetes mellitus, Type 2, Meta-analysis
Introduction
Pancreatic cancer mainly refers to pancreatic exocrine adenocarcinoma that has the characteristics of high malignancy, rapid development, and poor prognosis [1]. In the United States, 43,920 pancreatic cancer patients are diagnosed each year, resulting in approximately 37,390 deaths [2]. Pancreatic cancer is the fourth most common cause of cancer death worldwide [1,3]. Radical surgery is one of the treatment methods for pancreatic cancer [4]. However, early diagnosis is difficult with low general surgical resection rate from 10% to 30% [4]. Chemotherapy may improve the quality of life but not the long-term survival [5]. Radiotherapy can relieve the pain, but not other symptoms [6]. Smoking, excessive drinking, chronic pancreatitis and family history of cancer are widely recognized as potential risk factors for pancreatic cancer [7]. In addition, epidemiological studies have shown that diabetes, especially non-insulin-dependent diabetes mellitus (Type 2 Diabetes, T2DM) can increase the risk of pancreatic cancer [2,8,9]. In recent years, the incidence of pancreatic cancer in T2DM patients significantly increased, and the prognosis is poor in most patients because of difficulty in early diagnosis, high degree of malignancy, rapid development and lack of treatment with 5-year survival rate of less than 5% [10].
Therefore, it is of great importance to develop the potential drugs for the prevention and treatment of pancreatic cancer in T2DM patients. Metformin is one of the recommended firstline drugs in the treatment of T2DM. Its effects are acted mainly through reducing insulin resistance, improving the hypoglycemic effect by insulin and reducing blood sugar in T2DM patients [11]. It has been found that metformin may not only lower blood glucose in T2DM, but also significantly reduce the malignancy rates, including pancreatic cancer, lung cancer, esophageal cancer, colon cancer, liver cancer, and breast cancer [12-14]. Two studies in the United States have shown that metformin significantly reduced the risk of pancreatic cancer in T2DM patients treated with metformin compared with those without [15,16]. It is reported [17] that metformin inhibited the growth of pancreatic cancer cells by down-regulating the activity of insulin-like growth factor 1 signaling pathway. It is also shown [18] that metformin inhibited the activity of respiratory chain complex I to reduce the synthesis of ATP, thereby increasing AMP/ATP ratio, activating of AMPK pathway, and the DNA synthesis and inhibiting cell proliferation of pancreatic cancer cell. Lee et al. [19,20] showed that there were no differences between metformin and other hypoglycemic drugs in reducing the incidence of pancreatic cancer. However, many epidemiological studies [21,22] have shown the role of metformin in the prevention of pancreatic cancer. Therefore, whether metformin can reduce the risk of pancreatic cancer is still controversial.
In this meta-analysis, the relationship between metformin and pancreatic cancer in T2DM patients was investigated. Our findings may provide reliable evidence for further clinical prevention and treatment of pancreatic cancer in T2DM patients.
Materials and Methods
Inclusion and exclusion criteria
We included studies that met the following criteria: 1) published studies on the relationship between metformin and pancreatic cancer in T2DM patients; 2) similar study aims and statistical methods with complete data; and 3) cohort study or case-control study. In the cohort study, the metformin group was treated with metformin, and the control group was treated with other antidiabetic drugs (including sulfonylureas, thiazolidinediones, or insulin). In the case-control study, case group were T2DM patients with pancreatic cancer, control group were T2DM patients without pancreatic cancer, and exposure was metformin.
We excluded studies that met the following criteria: 1) flawed study design flaws with incomplete data; 2) exposure factors were not metformin; 3) no evaluation of relationship between metformin and pancreatic cancer; or 4) duplicates.
Search strategy
The terms, including “metformin”, “biguanides”, “antidiabetic”, “diahetes”, “diahetes mellitus”, “abnormal glucose metabolism”, “hyperglycemia”, “pancreatic cancer”, “pancreatic tumor”, “pancreatic adenocarcinoma”, “case-control study”, “risk”, “incidence” and “prevalence”, were searched on PubMed, Embase, Cochrane Library, Highwire, China Biology Medicine (CBM), China Knowledge Resource Integrated Database (CNKI), Wanfang and VIP Journal Integration Platform, until September 2016. Search strategy of combined text and MeSH terms was performed depending on the requirement of databases. Clinical trials register websites and references of review papers were also reviewed for comprehensiveness.
Information extraction and evaluation
All articles were reviewed by two reviewers to independently screen titles and abstracts, and review the full-text of the eligible articles. Two reviewers extracted and compared information of all included articles, and evaluated their quality. When they disagreed with each other, disagreements were either discussed to reach a consensus between the two reviewers or decided by a third reviewer.
The extracted information included: first author, time of publication, type of study, exposure factors (use of metformin), Relative Risk (RR) and 95% Confidence Interval (CI), confounders, follow-up time. The corresponding authors were contacted by telephone or mails to provide supplemental information when essential data was lacking.
Studies were evaluated for methodological quality based on Newcastle-Ottawa Scale (NOS) score [23] in terms of selection of case and controls, comparability of cases and controls and ascertainment of exposure. The evaluations were performed by the two independent reviewers, and ≥ 7 points were considered as high-quality literature with the highest score of 9 points.
Statistical analysis
Meta-analysis was performed using RevMan5.2 (Cochrane Collaboration) [24]. Quantitative data used relative risk (RR) for efficacy analysis and categorical data used Weight Mean Difference (WMD), presented as 95% Confidence Interval (CI). χ2 test was used for heterogeneity analysis, and heterogeneity was assessed by I2. If P>0.1 and I2<50%, the fixed effects model was used; otherwise, the heterogeneity was assessed to determine whether random effects model can be used. If there was obvious statistical heterogeneity but clinical homogeneity among all studies or subgroups, random effect model was used. Sensitivity analysis was performed for each outcome. A funnel plot was used to detect the presence of publication bias.
Results
Characteristics of patients
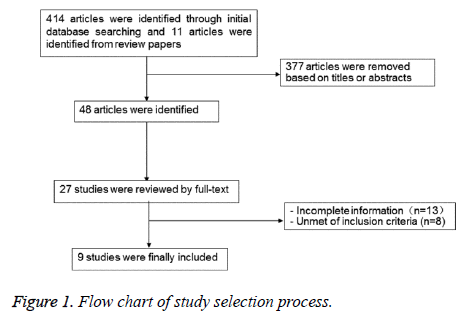
A total of 425 references were reviewed, and 9 studies were finally included after reviewing abstracts, inclusion and exclusion criteria. Seven of them [20,25-30] were cohort studies and two were [31,32] case-control studies. The characteristics of all included study were shown in Table 1, and the diagram of literature screening was shown in Figure 1.
| Studies | Study design | Country of study | Number of patients (T/C) | Age (years) | Length of follow up | Control group | Calibration parameters |
|---|---|---|---|---|---|---|---|
| Li [11] | Case-control | United States | 973 /863 | 61 | NR | Insulin | Age, sex, race, smoking, drinking, body mass index, family history of cancer |
| Currie [12] | Cohort | United Kingdom | 31421 /17506 | 62 | 2.42 | Sulfonylurea drugs; insulin | Age, sex, smoking, history of malignancy |
| Lee [13] | Cohort | United Kingdom | 11309 /22948 | ≥ 20 | 3.52 | Non-metformin | Age, gender, other oral anti-diabetic agents, CCI score, duration of metformin exposure |
| Van [14] | Cohort | United Kingdom | 109708/ 122403 | 63 | 9 | Non-metformin | Age, gender, body mass index, smoking, glycosylated haemoglobin, hospitalization 1 year prior to follow-up, acute coronary syndrome, hypertension, hyperlipidemia, stroke |
| Hsieh [15] | Cohort | Taiwan | 61777 /677378 | 61.4 | 8 | Sulfonylurea drugs; insulin | Age, gender |
| Bodmer [16] | Case-control | United States | 2763 /16578 | 69.5 | Not applicable | Non-metformin | Age, sex, BMI, smoking, alcohol consumption, diabetes duration |
| Liao [17] | Cohort | United States | 49803 /199212 | 55.9 | 6.3 | Sulfonylurea drugs; insulin | Chronic pancreatitis, age, gallstones, hepatitis B |
| Ruiter [18] | Cohort | Taiwan | 52698/32591 | ≥ 18 | 2.8 - 4.6 | Sulfonylurea drugs | Age, sex, duration of hyperglycemia, use of other drugs, hospitalization before follow-up, dosage |
| Tsilidis [19] | Cohort | Netherlands | 51484/18264 | 35 - 90 | 5.1 | Sulfonylurea drugs | Age, sex, body mass index, smoking, drinking, use of nonsteroidal anti-inflammatory drugs, statins and hormone drugs |
| T: Treatment; C: Control; NR: Not Report; CCI: Complications of Composite Index. | |||||||
Table 1: Baseline characteristics of included studies.
Quality evaluation
NOS score was used to evaluate the quality of all studies. As shown in Table 2, the NOS score suggested that the included studies were of high quality.
| Included studies | 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Li [11] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Currie [12] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Lee [13] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Van [14] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Hsieh [15] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Bodmer [16] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Liao [17] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Ruiter [18] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Tsilidis [19] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 1. Representativeness of the cohort; 2. Control from the same cohort; 3. Quality of exposures; 4. Occurrence of end-point events; 5A. Control of age; 5B. Control of other confounders; 6. Quality of end-point events; 7. Length of follow-up; 8. Definition of cohort. | ||||||||||
Table 2: Risk assessment for included studies.
Comparison of metformin and non-metformin drugs
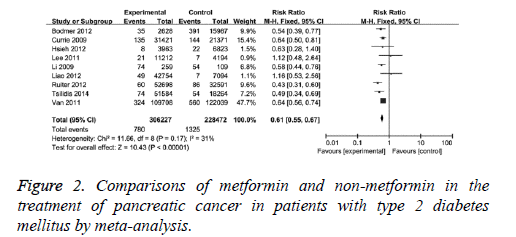
The effects of metformin and non-metformin drugs on pancreatic cancer were compared. The comparisons were performed in nine clinical studies with mild statistical heterogeneity (I2=31%) among studies, therefore, fixed-effect model was used. It showed that the adjusted RR was 0.61 (95% CI (0.55, 0.67), P<0.001) for T2DM patients with metformin compared with those without, as in Figure 2. When 2 case-control studies were excluded, the revised RR was 0.62 (95% CI (0.56, 0.680, P<0.001) for T2DM patients with metformin compared with those without. This result indicates metformin has better protection effect than non-metformin drugs on pancreatic cancer.
Comparison of metformin and sulfonylurea drugs
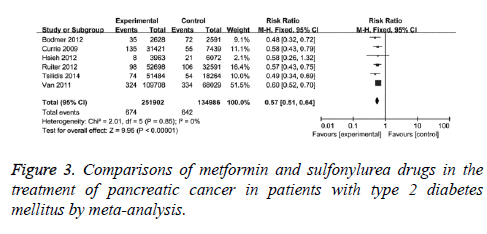
The effects of metformin and sulfonylurea drugs on pancreatic cancer were also compared. The comparisons were performed in 6 clinical studies with no statistical heterogeneity (I2=0), therefore, fixed-effect model was used. It showed that the adjusted RR was 0.57 (95% CI (0.51, 0.640, P<0.001) for T2DM patients with metformin compared with those with sulfonylurea drugs, as in Figure 3. When case-control studies were excluded, the revised RR was 0.58 (95% CI (0.52, 0.65), P<0.001) for T2DM patients with metformin compared with those with sulfonylurea drugs, indicating that the protection effect of metformin on pancreatic cancer is better than sulfonylurea drugs.
Comparison of metformin and insulin
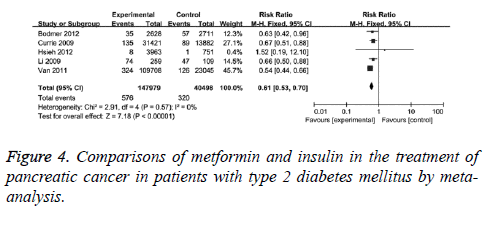
Furthermore, the differences in protection effect on pancreatic cancer between metformin and insulin were compared. The comparisons were performed in 5 clinical studies with no statistical heterogeneity (I2=0), therefore, fixed-effect model was used. It showed that the adjusted RR was 0.61 (95% CI (0.53, 0.70), P<0.001) for T2DM patients with metformin compared with those with insulin, as in Figure 4. When case-control studies were excluded, the revised RR was 0.59 (95% CI (0.50, 0.70), P<0.001) for T2DM patients with metformin compared with those with insulin. This data suggests the protection effect of metformin.
Discussion
The 2012 China Cancer Registration Annual Report showed that pancreatic cancer has been ranked ninth in the incidence of all malignant tumors in China with incidence rate of 8.19/10 million [33]. American Cancer Society shows that [34] the number of pancreatic cancer deaths per year is about 34,000, and 5-year survival rate is only 33%. Currently, pancreatic cancer has become one of the malignant tumors with highest mortality in China [35].
Epidemiological studies have shown that T2DM can increase the risk of pancreatic cancer [21,22,32]. For example, Ben et al showed that the risk of pancreatic cancer was 1.94 times higher in T2DM patients compared with those without (RR: 1.94, 95% CI (1.66, 2.27)) [8]. Batty [36] showed in a large cohort study that fasting hyperglycemia may increase the incidence of pancreatic cancer and associated mortality. Epidemiologic studies showed that metformin may reduce the incidence of pancreatic cancer in diabetic patients [25,31]. Metformin as an insulin sensitizer can inhibit gluconeogenesis and glycogen breakdown, promote muscle glucose uptake, and effectively reduce blood glucose [37]. Studies [38,39] have also shown that metformin can inhibit the proliferation of pancreatic cancer cells through AMPK/mTOR axis, insulin/insulin-like growth factor signaling pathway, and induction of cell cycle arrest [23].
This study showed that Metformin could effectively reduce the risk of pancreatic cancer in patients with diabetes when compared with sulfonylureas, insulin and other non-metformin drugs from analysis of nine high-quality clinical studies. Metformin was associated with a 61%, 57% and 61% decrease in risk compared with non-metformin, sulfonylureas and insulin. The sensitivity analysis showed that the results were stable. In a meta-analysis, Wang et al. [40] showed that metformin may reduce the risk of pancreatic cancer in T2DM patients. However, the meta-analysis was limited in that 1) it included both cohort study and case-control study with completely different data extracted from the treatment and control group; 2) two studies (Currie et al. [25] and Little et al. [41]) reported no data for RR calculation; and 3) incomplete literature review with low NOS score after 2012. Yu et al. [42] showed that metformin reduced the risk of pancreatic cancer by 51% and 53%, respectively, compared with sulfonylurea and insulin group; however there were no analysis of case-most obvious (normalized relative risk of incidence SRR=0.54, 95% CI 90.35, 0. 830; mortality SRR=0.64, 95, 95% CI (0.48, 0.86)). However, in their study, subgroup comparisons with other specific hypoglycemic agents were not analysed. Sadeghi et al. [44] showed that the use of metformin was associated with an increased survival rate in T2DM patients with pancreatic cancer. Consistently, we found that metformin had protection effects on pancreatic cancer in T2DM patients. Furthermore, the literatures included in this paper were of high quality and published in the near 10 years with NOS score of 7 to 9 points, indicating the robustness of the conclusions.control studies. In a meta-analysis of relationship between metformin use and cancer [43], it showed that metformin may reduce the incidence and mortality of multiple malignancies, including liver, pancreas, colon and lung cancers. The reduction in incidence and mortality of pancreatic cancer was
However, there are still some limitations in this study. Only a few cohort studies were included, and some of the literature did not provide detailed information on age, gender, dosage of metformin and other hypoglycemic agents, and length of use. Therefore, the above calibration parameters cannot be excluded. In addition, most of the included studies were in English language, indicating language bias. Calibration parameters including smoking, alcohol, race, region, and pancreatic cancer lesions were not analysed, introducing potential bias of this study. Last but not least, clinical randomized controlled trials were not identified.
In conclusion, this study analysed 9 high quality studies and showed metformin treatment reduced the risk of pancreatic cancer in patients with type 2 diabetes, when compared with sulfonylurea or insulin. Our findings may shed light on the treatment of pancreatic cancer in patients with type 2 diabetes.
Acknowledgements
This work was supported by General project of Hangzhou Health Science and Technology project (No. 2015A53).
Conflict of Interest
The authors declare no conflict of interests.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10-29.
- US Cancer Statistics Working Group. Unites States Cancer Statistics 1999-2007 incidence and mortality web-based report. Atlanta: US Department of Health and Human.
- Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, Maisonneuve P, Sebastiano PD. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol 2012; 19: 1644-1662.
- Michalski CW, Weitz J, Buchler MW. Surgery insight: surgical management of pancreatic cancer. Nat Clin Pract Oncol 2007; 4: 526-535.
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW, European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350: 1200-1210.
- Sunamura M, Egawa S, Fukuyama S, Motoi F, Takeda K. Chemotherapy for pancreatic cancer. Gan To Kagaku Ryoho 2003; 30: 1901-1908.
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006; 20: 197-209.
- Ben Q, Xu M, Ning X, Liu J, Hong S. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011; 47: 1928-1937.
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005; 92: 2076-2083.
- Davis JL, Pandalai PK, Ripley RT, Langan RC, Avital I. Expanding surgical treatment of pancreatic cancer: the role of regional chemotherapy. Pancreas 2012; 41: 678-684.
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334: 574-579.
- Li D1, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137: 482-488.
- Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010; 33: 322-326.
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304-1305.
- Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010; 3: 1451-1461.
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012; 7: e33411.
- Karnevi E, Said K, Andersson R, Rosendahl AH. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signaling pathway in human pancreatic cancer cells. BMC Cancer 2013; 13: 235-246.
- Sinnett-Smith J, Krisztina K, Kui R, Rozengurt E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun 2013; 430: 352-357.
- Little MW, Pugh TF, Carey FJ, Ndokera R, Ing H, Robinson RJ, Dennison AR, Metcalfe MS, Clark A, Hart AR. The potential protective effect of metformin against pancreatic cancer: preliminary results from a case-control study in two UK centres. Gut 2011; 60: 78-79.
- Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800 000 individuals. BMC Cancer 2011; 11: 20.
- Yu H, Yin L, Jiang X, Sun X, Wu J. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One 2014; 9: e116327.
- Duncan BB, Schmidt MI. Metformin, cancer, alphabet soup, and the role of epidemiology in etiologic research. Diabetes Care 2009; 32: 1748-1750.
- Pollak M. Metformin and pancreatic cancer: a clue requiring investigation. Clin Cancer Res 2012; 18: 2723-2725.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539-1558.
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009; 52: 1766-1777.
- Van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose lowering agents and the patterns of risk for cancer: a study with the general practice research database and secondary care data. Diabetologia 2012; 55: 654- 665.
- Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res 2012; 2012: 413782.
- Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. Gastroenterol Hepatol 2012; 27: 709-713.
- Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012; 35: 119-124.
- Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, Sacerdote C, Ashby D, Vineis P, Tzoulaki I, Ioannidis JP. Metformin does not affect cancer risk: a cohort study in the U.K. clinical practice research data link analysed like an intention-to-treat trial. Diabetes Care 2014; 37: 2522-2532.
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009; 137: 482-488.
- Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocr Relat Cancer 2012; 19: F9-9F26.
- Wu C, Miao X, Huang L, Che X, Jiang G, Yu D, Yang X, Cao G, Hu Z, Zhou Y, Zuo C, Wang C, Zhang X, Zhou Y, Yu X, Dai W, Li Z, Shen H, Liu L, Chen Y, Zhang S, Wang X, Zhai K, Chang J, Liu Y, Sun M, Cao W, Gao J, Ma Y, Zheng X, Cheung ST, Jia Y, Xu J, Tan W, Zhao P, Wu T, Wang C, Lin D. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet 2011; 44: 62-66.
- Olowokure O, Qi X. Pancreatic cancer: Current standards, working towards a new therapeutic approach. Expert Rev Anticancer Ther 2014; 14: 495-497.
- Kai Gu, Wu CX, Bao PP, Wang CF. Incidence of pancreatic cancer in shanghai: a current, retrospective and comparative exploration. J Surg Concepts Pract 2009; 14: 510-515.
- Batty GD, Shipley MJ, Marmot M, Smith GD. Diabetes status and post-load plasma glucose concentration in relation to site-specific cancer mortality: findings from the original Whitehall study. Cancer causes control 2004; 15: 873-881.
- Nasri H, Rafieian-Kopaei M. Metformin: Current knowledge. J Res Med Sci 2014; 19: 658-664.
- Fuhrmeister IP, Branchini G, Pimentel AM, Ferreira GD, Capp E, Brum IS, von Eye Corleta H. Human granulosa cells: insulin and insulin-like growth factor-1 receptors and aromatase expression modulation by metformin. Gynecol Obstet Invest 2014; 77: 156-162.
- Han G, Gong H, Wang Y, Guo S, Liu K. AMPK/mTOR-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol Ther 2015; 16: 77-87.
- Wang Z, Lai ST, Xie L, Zhao JD, Ma NY. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014; 106: 19-26.
- Little MW, Pugh TF, Carey FJ, Rdokera R, Ing H, Robinson R, Dennison AR, Metcalfe MS, Clark A, Hart AR. The potential protective effect of metformin against pancreatic cancer: preliminary results from a case -control study in two UK centres. Gut 2011; 60: 78-79.
- Yu X, Huang QY, Tang SH. Relation between metformin and the risk of pancreatic cancer in type 2 diabetes patients: a meta-analysis. Chinese General Practice 2016; 19: 195-198.
- Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol 2013; 37: 207-218.
- Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res 2012; 18: 2905-2912.