Research Article - Biomedical Research (2017) Volume 28, Issue 4
Relationship between the poor glycemic control and risk factors, life style and complications
Yusuf Kayar1*, Aysegul Ilhan2, Nuket Bayram Kayar3, Nurcan Unver4, Ganime Coban4, Iskender Ekinci1, Jamsid Hamdard1, Ozgul Pamukcu2, Hatice Eroglu21Division of Gastroenterology, Department of Internal Medicine, Bezmialem Vakif University, Istanbul, Turkey
2Department of Internal Medicine, Sisli Etfal Education and Research Hospital, Istanbul, Turkey
3Department of Family Medicine, Bagcilar Education and Research Hospital, Istanbul, Turkey
4Division of Pathology, Bezmialem Vakif University, Istanbul, Turkey
- *Corresponding Author:
- Yusuf Kayar
Department of Internal Medicine
Bezmialem Vakif University, Turkey
Accepted on August 19, 2016
Abstract
It has shown that the decrease of blood glucose levels in patient with diabetes mellitus decreases mortality and morbidity rates. Main purpose in diabetes is to achieve and prevent the glycemic control. We aimed to evaluate the relationship between poor glycemic control and metabolic parameters, individual life and complications. Seven hundred fifty seven patients with type II diabetes mellitus have evaluated with demographical characteristic, body mass index, abdominal circumferences, blood pressures, dietary compliances, physical exercise statuses and laboratory analysis; and the relationship of these parameteres were investigated. Poor glycemic control was found significantly associated with duration of diabetes, age of onset, family history, job status, educational status, antidiabetic drugs, body mass index, abdominal circumference, hypertension, lipid and fasting plasma glucose levels. There was a significant relationship between the glycemic control and dietary compliance, physical activity, self blood glucose monitoring and drug compliance. While there is a significant relationship between the poor glycemic control and nephropathy, retinopathy, neuropathy and cardiovascular diseases; no significant relationship was seen in the cerebrovascular diseases and arthropathy. We have pointed the relationship of glycemic control with sociodemographic, medical status, life style, lipid levels and complications. Better results can be obtained by eliminating the factors related to poor glycemic control.
Keywords
Hyperglycemia, Life style, Complications, Risk factors.
Introduction
Diabetes Mellitus (DM) prevelance of which is growning gradually is one of the major causes of morbidity and mortality worldwide. DM is the most common chronical illness at adults. It is estimated that 300 million people will have DM by 2025 and it will reach approximately 439 million and the prevelance is estimated as 7.7% by 2030. It is anticipated that 3.96 millions of patients with DM will die annually related to the diabetes and it will compromise 6.8% of all the causes of death [1-3]. Awareness of DM in Turkey is still quite poor as a developing country. The decrease of blood glucose levels in patient with DM decreases the mortality and morbidity rates significantly. The main purpose in patients with DM is to achieve and to prevent the glycemic control. In randomised prospective clinical trials and epidemiologic studies, it has shown that microvascular and macrovascular complications are reduced by obtaining the glycemic control. Achieving the glycemic control is the most important issue to prevent the organ damage and not to occur other complications of DM [4].
Although the glycemic control is the main target of the treatment, it is not been provided at majority of the diabetic patients. It is quite difficult to obtain the glycemic control clinically. Because there are several genetical and environmental factors such as age, sex, educational status, body mass index (BMI), duration of diabetes, life style and family history, which are related to poor glycemic control [5,6]. The objective of the present study was to examine the relationship between poor glycemic control and metabolic parameters, individual life style and complications of patients with type 2 DM.
Materials and Method
The study included 757 patients who were undergoing treatment and follow-up by Sisli Etfal Education, Research Hospital Internal Medicine Department and Bezmialem Vakıf University Internal Medicine Department, with diagnosis of type 2 DM were aged 18-70 years. Our study was designed as a sectional observational study. We included patients were admitted to our hospital with the diagnosis of type 2 DM between August 2013 and September 2015. The diabetic patients who were diagnosed as type 1 diabetes mellitus were excluded.
Clinical measurements
Demographic information (i.e., age and gender) was documented for all patients. Height (m) and weight (kg) measurements were obtained to calculate the BMI. BMI was calculated by dividing weight in kilograms by the square of height in meters. Waist circumference (cm) was measured midway between the lower rib margin and the iliac crest. Systolic and diastolic blood pressures were measured using an automatic sphygmomanometer with an appropriate cuff size on the right arm after a resting period of 10 min. Patients were diagnosed as DM according to the criteria of American Diabetes Association (ADA). Duration of the illness and DM disease onset age were documented. Patients whose systolic/ diastolic blood pressure is ≥ 140/90 mmHg or who is on an antihypertensive treatment were accepted as hypertensive patients [7]. Patients whose BMI is <25 kg/m2 were considered as normal, 25-29 kg/m2 as overweight and ≥ 30 kg/m2 as obese [8].
Life style measurements
Furthermore, dietary compliance, physical exercises, blood glucose monitoring at home and medicine compliance have been questionized. Smoking, alcohol consumption and patients’ medical treatments for diabetes (only oral antidiabetics (OAD), only insulin or both) have been documented. Data on self-management behaviors included diet, exercise, and self-monitoring of blood glucose. These were collected to assess the adherence to diabetic control measures that included physical exercise, diet, and blood glucose testing [9]. Self-management behaviors were assessed using the Summary of Diabetes Self-Care Activities Measure scale [10], which contains eleven question items designed to ask the patients about their diabetes self-care activities during the past 7 days. If patients were sick during the past 7 days, they were asked to think back to the last 7 days that they were not sick. Medical adherence was determined by self-reporting with the use of the eight-item Morisky scale [11]. The scale contains questions asking the patient to respond “yes” or “no” to a set of eight questions. A positive response indicates a problem with adherence. Therefore, higher scores indicate that a patient is least adherent to medications. In this study, a positive response was awarded one point and a negative response was awarded zero points. Patients were classified as highly and moderately adherent (score of 0-2), and least or not adherent (score of 3-8).
Laboratory measurements
Patients fasted after midnight and blood samples were drawn in the morning of the next day from an antecubital vein into vacuum tubes for laboratory tests, which were sent to the central laboratory. The levels of Hemoglobin A1c (HbA1c) and fasting glucose levels, lipid profiles (high density lipoprotein (HDL), low density lipoprotein (LDL), triglyceride, and cholesterol) were measured for each patient. Blood glucose and lipid profile were measured by enzymatic methods. HbA1c were measured by immunoturbidimetric method and high performance chromatography respectively and lipid profile was analyzed by automatic spectrophotometer. Patients whose HbA1c levels <7% were considered as with good glycemic control; and whose HbA1c ≥ 7% were considered as poor glycemic control [7]. Based on the values of ≥ 200 mg/dl for cholesterol; <50 mg/dL (women) and <40 mg/dL (men) for HDL; ≥ 100 mg/dL for LDL and ≥ 150 mg/dL for triglyceride; the patients whose values are higher of these values or who are on treatment in spite of the normal results were considered as dyslipidemic [7].
Complications measurements
All patients were examined for the presence of retinopathy by an ophthalmologist. Presence of nephropathy was evaluated in terms of urinalysis, 24-hour urine protein test and creatinine clearance. Glomerular filtration rate was calculated. Presence of neuropathy was assessed by orally questioning the patients for any complaints about burning, tingling, pain, jerks etc. in the extremities. Direct radiography and MRI was performed for the patients with joint pain for the presence of arthropathy. The patients were examined and their electrocardiograms were evaluated by a cardiologist and a cardiovascular surgeon for the presence of any cardiovascular disease. If necessary, the patients were further evaluated by using vascular ultrasound and coronary angiography. The patients were questioned for the presence of cerebrovascular disease by clinic and neurological examinations.
Ethics statement
All participants provided written consent for participation in the study. Ethics approval for conducting this study was received from the Ethical Committee of the Sisli Etfal Education and Research Hospital (Istanbul, Turkey). All procedures were in accordance with the ethical standards of the committee on human experimentation of our institution and with the Declaration of Helsinki.
Data analysis
The IBM SPSS 22 (IBM SPSS, Turkey) programme was used for the statistical analyses of data from this study. The relationship of glisemic control with demographic parameters, laboratory parameters, life style and complications was analyzed using chi-square, Mann-Whitney U test, and independent samples t-tests. Quantitative data were reported as percentages and mean ± standard deviation; normally distributed parameters were compared using Student’s t-tests and nonnormally distributed parameters were compared using Mann Whitney U tests. Qualitative data were compared using the chi-square test. A P value<0.05 was considered statistically significant.
Results
In this study, total of 757 patients with type 2 DM enrolled; including 405 (53.5%) women and 352 (46.5%) men. The mean age 57 ± 9.1 years for women, 55 ± 7.3 years for men and 56 ± 8.3 years for whole study population (range: 26-85 years). 67.5% of the patients had poor glycemic control (HbA1c was found as ≥ 7%) while only 32.5% had good glycemic control. Age of diabetes onset 49±10.8 (range: 20-83) years and duration of disease 7.5 ± 6.6 (range: 1-39 years) years. 91% of the patients were married, 70% do not work in any job and 78% have positive family history. 61% of patients were only on OAD therapy, while only 8% were on insulin and 31% were both on OAD + insulin. 10.9% of the patients were normal weight, while 89.1% of patients were overweight or obese. Total cholesterol in 53% of patients, triglyceride in 54% of patients, LDL-cholesterol in 74% of patients were elevated; while HDL level in 45% of patients was lower than normal. 59% of the patients had hypertension.
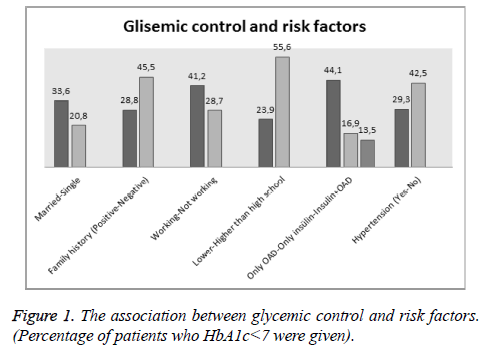
While poor glycemic control was not in relationship with age and sex, it was found significantly associated with duration of diabetes, age of onset, family history, job status, educational status, antidiabetic drugs chosen for treatment, BMI, abdominal circumference, presence of hypertension, cholesterol, tryglyceride, LDL, HDL and fasting plasma glucose levels. Poor glycemic control was found to be significantly higher in patients taking insülin therapy and in patients taking insülin+oral anti-diabetic medication than in patients taking only oral anti-diabetic medication. The association between poor glycemic control and demographical, clinical and antropometrical factors are shown in Figure 1 and Table 1.
| Variables | Total | HbA1c<7 | HbA1c ≥ 7 | P-value |
|---|---|---|---|---|
| Age | 56 ± 8.3 | 56.1 ± 11.1 | 56.5 ± 11.5 | 0.54 |
| Onset age of DM | 49 ± 10.8 | 51.3 ± 10.8 | 48 ± 10.7 | <0.001 |
| Sex | ||||
| Female | 405 | 135 (33.3%) | 270 (66.7%) | 0.59 |
| Male | 352 | 111 (31.5%) | 241 (68.5%) | |
| DM duration | 7.5 ± 6.6 | 4.7 ± 5.3 | 8.6 ± 6.8 | <0.001 |
| Marital Status | ||||
| Married | 690 | 232 (33.6%) | 458 (66.4%) | 0.034 |
| Single | 67 | 14 (20.8%) | 53 (79.2%) | |
| Family history | ||||
| Positive | 590 | 170 (28.8%) | 420 (71.2%) | <0.001 |
| Negative | 167 | 76 (45.5%) | 91 (54.5%) | |
| Job status | ||||
| Working | 228 | 94 (41.2%) | 134 (58.8%) | 0.001 |
| Not working | 529 | 152 (28.7%) | 377 (71.3%) | |
| Educational Status | ||||
| Lower than high school | 552 | 132 (23.9%) | 420 (76.1%) | <0.001 |
| Higher than high school | 205 | 114 (55.6%) | 91 (44.4%) | |
| Diabetic medicine | ||||
| Only OAD | 462 | 204 (44.1%) | 258 (55.9%) | - |
| Only insülin | 59 | 10 (16.9%) | 49 (83.1%) | <0.001 |
| Insulin+OAD | 236 | 32 (13.5%) | 204 (86.5%) | <0.001 |
| Hypertension | ||||
| Yes | 447 | 131 (29.3%) | 316 (70.7%) | <0.001 |
| No | 310 | 132 (42.5%) | 178 (57.5%) | |
| Body mass index | 30.4 ± 5.7 | 29.8 ± 6.06 | 30.7 ± 5.5 | 0.035 |
| Abdominal circum* | 95.1 ± 11 | 92.9 ± 11.9 | 96.1 ± 12.3 | <0.001 |
| Cholesterol | 209 ± 47 | 202 ± 44.4 | 212 ± 48.7 | 0.007 |
| Triglyceride | 187 ± 151 | 154 ± 80 | 202 ± 173 | <0.001 |
| Low density lipoprotein | 125 ± 39 | 120 ± 39.7 | 128 ± 39.6 | 0.014 |
| High density lipoprotein | 47.6 ± 13 | 49 ± 12.9 | 46 ± 13.9 | 0.007 |
OAD: Oral Antidiabetic Circum; *: circumference; DM: Diabetes Mellitus
Table 1. The association between poor glycemic control and demographical, clinical and antropometrical factors.
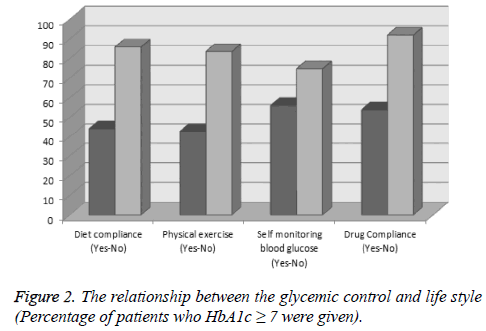
It has seen that while 44% of the patients have dietary compliance, 56% has not; and while 39.2% of the patients stated that they do regular physical activity, 60.8% of the patients do not. While 64.1% of the patients have compliance to medical treatment, it has seen that 35.9% of the patients do not take their drugs regularly and only 39.1% of patients regularly do self-blood glucose monitoring at home. In terms of the relationship between the poor glycemic control and individual lifestyle, it was seen that there is a significant relationship between the glycemic control and dietary compliance, regular physical activity, self-blood glucose monitoring at home and drug compliance (Figure 2 and Table 2).
| Variable | Total | HbA1c<7 | HbA1c ≥ 7 | P-value | |
|---|---|---|---|---|---|
| Diet compliance | Yes | 333 | 186 (55.8%) | 147 (44.2%) | <0.001 |
| No | 424 | 60 (14.1%) | 364 (85.9%) | ||
| Physical exercise | Yes | 297 | 170 (57.2%) | 127 (42.8%) | <0.001 |
| No | 460 | 76 (16.5%) | 384 (83.5%) | ||
| Self monitoring blood glucose | Yes | 296 | 130 (43.9%) | 166 (56.1%) | <0.001 |
| No | 461 | 116 (25.1%) | 345 (74.9%) | ||
| Drug Compliance | Yes | 485 | 224 (46.1%) | 261 (53.9%) | <0.001 |
| No | 272 | 22 (8.1%) | 250 (91.9%) | ||
Table 2. The relationship between the glycemic control and dietary compliance, physical activity, self-blood glucose monitoring and drug compliance.
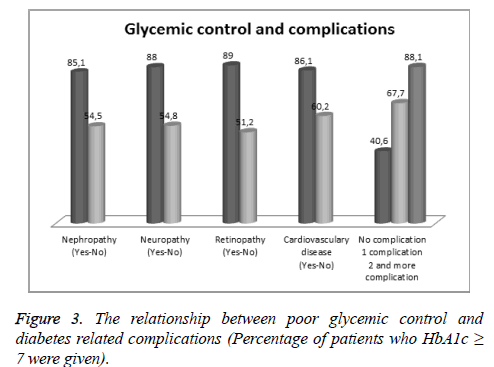
While in 35.8% of the patients, there was no complication related to diabetes; one complication was detected in 17.6% and in 46.6% of the patients more than one complication has been determined. When the association between the complications and poor glycemic control is considered, it has seen that while there is a significant relationship between the poor glycemic control and nephropathy, retinopathy, neuropathy and cardiovascular diseases; it was not significant for the cerebrovascular diseases, arthropathy and amputation. Furthermore, it was noticed that the worse glycemic control rates were detected, the more number of complications were seen. When patients with no complication were taken into account, poor glycemic control was significantly high in patients with one complication. And poor glycemic control was significantly higher in patients with 2,3 or more complication than in patients with one or no complication (Figure 3 and Table 3).
| Variables | Total | HbA1c<7 | HbA1c ≥ 7 | P value |
|---|---|---|---|---|
| Nephropathy | ||||
| Yes | 322 | 48 (14.9%) | 274 (85.1%) | <0.001 |
| No | 435 | 198 (45.5%) | 237 (54.5%) | |
| Neuropathy | ||||
| Yes | 291 | 35 (12%) | 256 (88%) | <0.001 |
| No | 466 | 211 (45.2%) | 255 (54.8%) | |
| Retinopathy | ||||
| Yes | 327 | 36 (11%) | 291 (89%) | <0.001 |
| No | 430 | 210 (48.8%) | 220 (51.2%) | |
| Cardiovasculary | ||||
| Yes | 215 | 30 (13.9%) | 185 (86.1%) | <0.001 |
| No | 542 | 216 (39.8%) | 326 (60.2%) | |
| Serebrovasculary | ||||
| Yes | 18 | 3 (16.7%) | 15 (83.3%) | 0.14 |
| No | 739 | 243 (32.9%) | 496 (67.1%) | |
| Artropathy | ||||
| Yes | 33 | 9 (27.2%) | 24 (72.8%) | 0.51 |
| No | 724 | 237 (32.7%) | 487 (67.3%) | |
| Amputation | ||||
| Yes | 6 | 2 (33.3%) | 4 (66.7%) | 0.96 |
| No | 751 | 244 (32.4%) | 507 (67.6%) | |
| Number of Complication | ||||
| No complication | 271 | 161 (59.4%) | 110 (40.6%) | - |
| 1 complication | 133 | 43 (32.3%) | 90 (67.7%) | <0.001 |
| 2 and more complication | 353 | 42 (11.9%) | 311 (88.1%) | <0.001 |
Table 3. The relationship between poor glycemic control and diabetes related complications.
Discussion
DM is one of the most important diseases of this era which is complicated, hard to control and increasing in numbers gradually. Several environmental and genetical factors such as family history, medical status, obesity, sociodemographic features, life style play role in glycemic control. Majority of the patients cannot reach the targeted HbA1c levels as we have shown in our study. EURIKA study which includes the data from 12 European countries showed that only 36% of type II diabetic patients obtain the targeted value [12]. In the study performed in Brasil, HbAc level less than 7 is obtained in 36% of patients [13]. Similar to European countries and Brasil, our study showed that the targeted value of HbA1c<7, was achieved low percentage in Turkish population as well.
In our study it has been shown that the disease onset younger were related to poor glycemic control. In the meta-analysis of ten studies, it has shown that glycemic control is better at patients who are 60 years or older [14]. We suppose that the reason of poorer glycemic control in younger populations compared to elders can be associated with the fact that the young people do not pay importance to their treatments as elders. As like the other reported studies, we have also found a significant relationship between the poor glycemic control and the duration of disease [5,15,16]. Longer duration of diabetes is known to be associated with poor control, possibly because of progressive impairment of insulin secretion with time because of β cell failure, which makes the response to diet alone or oral agents unlikely [17]. When the relationship between the poor glycemic control and medical status is investigated in Turkish population, as parallel to several previous studies, poor glycemic control levels of the patients who is on insülin and oral antidiabetic treatment concurrently have found significantly higher [5,15]. This may be related to the fact that patient treated by insulin or combination therapy have more severe disease that requires more aggressive treatment to control their disease, while patients with milder disease are more easily controlled by diet or oral hypoglycaemic agents.
In parallel with our study, it was also shown that there is a significant correlation between the poor glycemic control and being single, unemployments, patients and/or the patients who completed only primary education and patients with positive family history [15,16]. It is thought that better glycemic control in patients with higher educational status and laboring patients is about the awareness of the disease. Furthermore, better glycemic control in married patients can be related to the support of their partners. As similar to the study performed by Almutairi et al., in our study we have shown that 77.9% of the patients had a positive family history and this was associated with the poor glycemic control [16]. Furthermore, there was detected significant relationship between the poor glycemic control and obesity, abdominal circumference, fasting glucose and lipid levels. There is also some other reports indicating the correlation of glycemia with obesity and abdominal circumference [4,16]. Khattab et al., has stated in his study that there was a significant relationship between glycemic control and dyslipidemia, total cholesterol, tryglyceride and low density lipoprotein as similar to our study [5].
Previous studies showed relationship between glycemic control and life style [5,18]. In our study, we have also seen significant relationship between glycemic control and diet, exercise,medical compatibility and self blood glucose monitoring at home. It is obvious that awareness and compatibility of the patients to the disease and life style modification is very important in terms of controlling diabetes. Also, only a small number of diabetic patients are on regular diet and exercise programme. We suppose that life style changes can be improved by providing the awareness of the disease. In our study, we detected a significant relationship of poor glycemic control with microvascular and macrovascular complications and their numbers. Similar to the study of Almutairi et al., we have determined that the worse glycemic control is, the more number of complications are seen [16]. Improving glycemic control improves microvascular outcomes, as illustrated by the findings of a meta-analysis of randomized trials (25,760 participants) [19]. There was a reduction in the risk of microvasculary complications in the intensive compared with standard glycemic control group.
Type 2 diabetes, a chronic degenerative disease of epidemic proportions, is one of the major challenges to public health in the world. Although effective interventions to reduce the longterm complications are available, the complex interventions required and the size of the diabetic population have made the application of such therapies problematic [20]. For this reasons early and aggressive treatment modalities are recommended. Glucose control remains a major focus in the management of patients with type 2 diabetes. However, this should always be in the context of a comprehensive cardiovascular risk factor reduction program which include smoking cessation and adoption of healthy lifestyle habits, blood pressure control, lipid management and, in some circumstances, antiplatelet therapy [21]. Diet and exercise in the treatment of all stages should be in the forefront. Ideal and personalized medical nutrition therapy applicable though out the life should be planned. While in the recent past only lifestyle changes was the cornerstone treatment of type-2 DM, nowadays the medical treatment is recommended because of insufficiences of nonmedical approaches. In addition to lifestyle changes, current treatment for type-2 DM includes oral antidiabetic agents, insulin or any combination of the above. For the success of stepwise treatment of type-2 DM, drug choices should be based on the different pathophysiological processes. In summary, the effective treatment for type-2 DM depends on the balance between lifestyle changes, diet, exercise and personalized medical treatment. A healthy communication between the patient and health team is of great importance.
Conclusion
In the conclusion, we have shown the relationship of glycemic control with sociodemographic, medical status, life style, lipid levels and complications. Diabetes is a disease which is affected by several enviromental and genetical factors and require multidisciplinary approach for glycemic control. Successfull results can be obtained solely by eradication of the factors (job satatus, marital status, educational status, diabetic medicine treatment, hypertension, BMI, obesity, hyperlipidemia and life style) related to poor glycemic control. To achieve this aim, enhancement of awareness of patients about the disease and providing the life style modifications must be targeted initially.
References
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025. Diabetes Care 1998; 21: 1414-1431.
- Shaw JE, Sucre RA, Zimmet PZ. Global estimates for the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4-14.
- Roglic G, Unwin N. Mortality attributable to diabetes: Estimates for the year 2010. Diabetes Res Clin Pract 2010; 87: 15-19.
- Ghazanfari Z, Niknami S, Ghofranipour F, Larijani B, Agha-Alinejad H, Montazeri A. Determinants of glycemic control in female diabetic patients: a study from Iran. Lipids Health Dis 2010; 11: 79-83.
- Khattaba M, Khader YS, Al-Khawaldehd A, Ajlounid K. Factors associated with poor glycemic control among patients with Type 2 diabetes. J Diabetes Complicat 2010; 24: 84-89.
- Hartz A, Kent S, James P, Xu Y, Kelly M, Daly J. Factors that influence improvement for patients with poorly controlled type 2 diabetes. Diabetes Res Clin Pract 2006; 74: 227-232.
- American Diabetes Association. Standards of Medical Care in Diabetes 2015. Diabetes Care 2015; 38: 58-67.
- World Health Organization. Physical status: The use and interpretation of anthropometry: Report of a WHO Expert Committee. Technical report series 854. Geneva; 2005
- Davison KA, Negrato CA, Cobas R. Relationship between adherence to diet, glycemic control and cardiovascular risk fac-tors in patients with type 1diabetes: a nationwide survey in Brazil. J Nutrition 2014.
- Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Measure. Diabetes Care 2000; 23: 943-950.
- Morisky D, Green L, Levine D. Concurrent and predictive validity of a self reported measure of medication adherence. Med Care 1986; 24: 67-74.
- Banegas JR, Lopez-Garcia E, Dallongeville J, Guallar E, Halcox JP, Borghi C. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J 2011; 32: 2143-2152.
- Viana LV, Leitão CB, Kramer CK, Zucatti ATN, Jezini DL, Felício J. Poor glycemic control in Brazilian patients with type 2 diabetes attending the public health care system: a cross-sectional study. Br Med J 2013; 3: 3336.
- Sanal TS, Nair NS, Adhikari P. Factors associated with poor control of type 2 diabetes mellitus: A systematic review and meta-analysis. J Diabetology 2011; 3: 1-10.
- Adham M, Froelicher ES, Batiehaand A, Ajlouni K. Glycaemic control and its associated factors in type 2 diabetic patients in Amman, Jordan. East Mediterr Health J 2010; 16: 732-739.
- Almutairi MA, Said SM, Zainuddin H. Predictors of Poor Glycemic Control Among Type Two Diabetic Patients. Am J Med 2013; 3: 17-21.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet 1998; 352: 837-853.
- Benoit SR, Fleming R, Tsimikas AP, Ji M. Predictors of glycemic control among patients with Type 2 diabetes: A longitudinal study. BMC Public Health 2005; 5: 36.
- Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C. Targeting intensive glycemic control versus targeting conventional glycemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2011; 15: 8143.
- Nathan DM. Initial Management of Glycemia in Type 2 Diabetes Mellitus N Engl J Med 2002; 347: 1342-1349.
- Inzucchi SE, Bergenstal RM, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140-149.
- Raz I, Riddle MC, Rosenstock J. Personalized Management of Hyperglycemia in Type 2 Diabetes. Diabetes Care 2013; 36: 1779-1788.