Research Article - Biomedical Research (2017) Volume 28, Issue 13
Relationship between fat meal load-induced inflammation and atherosclerosis
Chunrong Jin1*, Xue Zhou2, Shuling Yang3, Xiaoli Zheng4 and Sijin Li5
1Department of Cardiology, the First Hospital of Shanxi Medical University, Taiyuan 030001, PR China
2Department of First Clinical Medicine, Shanxi Medical University, Taiyuan 030001, PR China
3Department of Cardiology, the People's Hospital of Jiaozuo City, Jiaozuo 454002, PR China
4Department of Cardiology, Hebei Yanda Hospital, Sanhe 065201, PR China
5Department of Nuclear Medicine, the First Hospital of Shanxi Medical University, Taiyuan 030001, PR China
- *Corresponding Author:
- Chunrong Jin
Department of Cardiology
The First Hospital of Shanxi Medical University, PR China
Accepted date: May 24, 2017
Abstract
We investigated the effect of fat meal load (FML) on inflammatory factors during the process of atherosclerosis (ATH). Ten male New Zealand white rabbits (fed with high-fat meal for 12 weeks, and provided FML at the beginning (W1), as well as in the 4th, 8th, and 12th weeks, (W4, W8, and W12, respectively) were used in this study. Blood samples were collected after fasting and at 2, 4, 6, and 8 h after FML (T0, T1, T2, T3, and T4, respectively) to detect plasma endothelin-1 and interleukin (IL)-6, IL-8, and IL-10; ATH conditions in the abdominal aorta were observed ultrasonically. At W12, the animals were sacrificed and ATH conditions were detected by hematoxylin and eosin staining. After continuous high-fat feeding, fasting plasma endothelin-1 was increased in W4, W8, and W12, IL-8 was increased in W4 and IL-10 was increased in W4 and W8. After FML, endothelin-1 was significantly increased at W0-T2, and W12-T1, IL-8 was increased at W0-T1-3, W4-T1-2, and W8-T1-3, and IL-10 was increased at W0-T1-4, W4-T2-4, and W12-T1-2. During early ATH, inflammation was severe and clearly observed at 4-6 h after FML.
Keywords
Inflammatory response, Fat meal load, Atherosclerosis
Introduction
Atherosclerosis (ATH) is a pathological process during which the arterial intima is thickened, the vascular wall becomes stiff, and atheromatous plaques appear locally, leading to stenosis or occlusion of the vascular cavity and corresponding clinical symptoms. ATH-induced cardiovascular and cerebrovascular diseases are the leading cause of human mortality worldwide. The occurrence and development mechanisms of ATH are very complicated. Since the inflammation hypothesis was proposed by Ross [1], an increasing number of researchers have considered ATH as a chronic inflammatory response based on endothelial dysfunctions. A recent clinical trial showed that some anti-inflammatory cardiovascular drugs reduced the incidence of major coronary events and total coronary events via their anti-inflammatory effects, but these drugs failed to meet the primary endpoints of reducing the risks of cardiovascular death, heart attack, and stroke [2]. Therefore, systematically determining the relationships between inflammations and ATH is essential for preventing and treating cardiovascular and cerebrovascular diseases.
High-fat meals may cause endothelial dysfunction and mild inflammation in the vessel walls [3,4], and postprandial blood fat disorders play important roles in the occurrence and development of ATH [5]. Giannattasio et al. [6] confirmed that a high-fat diet could cause acute hypertriglyceridemia as well as significantly damage endothelial functions; this damage was much more obvious in patients with existing lipid disorders. After consuming high-fat, high-carbohydrate diets, more intense and more durable inflammation was observed in obese subjects [7]. After one fat meal load (FML), healthy young men show reduced coronary blood flow [8]. In contrast, American society for nutrition once reported a review that presented healthy diets rich in lean fish, raw vegetables, fresh fruit, and poultry, with wine and fewer high-fat dairy products favorably slow the development of ATH by means of improving endothelial dysfunction and mild inflammation possibly [9], and reduce the incidence of cardiovascular events. However, its mechanisms of action are not clear and definite.
Previous studies have focused on comparing endothelial function and inflammatory factors in specific populations or at certain time point after consuming high-fat meals, while the dynamic changes in endothelial function and inflammatory factors during the process of ATH have not been widely examined. Endothelin 1 (ET-1) is a potent vasoconstrictor; interleukin 6 (IL-6) and IL-8 are classic inflammatory factors and are involved in the early formation of ATH [10-12], and IL-10 may downregulate inflammation, thus acting as a protective factor against ATH. In this study, FML was administrated at different stages of ATH and the changes of ET-1, IL-6, IL-8, and IL-10 before and after FML were analyzed. The goal of this study was to investigate the effect of FML on endothelial function and inflammatory responses during ATH progression and to provide a basis for clinical practice.
Materials and Methods
Subjects and establishment of ATH experimental model
Ten healthy male New Zealand white rabbits (3 months old, weighed 2.5-3 kg, purchased from the Shanxi Institute of Animal Husbandry and Veterinary) were fed with high-fat diets for 12 weeks, and a high-fat meal load was administrated at the beginning, as well as in the 4th, 8th, and 12th weeks. Refer to the following related literature, Hypercholesterolaemia and ATH can be triggered in a rabbit with more than 1-2% cholesterol [13], a high-fat diet given (contained 1% cholesterol, 8% egg yolk, and 91% basic feed) are to make rabbit atherosclerosis model, fat-load diet given (an additional 1% cholesterol, the amount of 2% cholesterolon) is the purpose of this test, on the study of the relationship between fat-load induced inflammation and atherosclerosis. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the First Hospital of Shanxi Medical University.
Detection of plasma ET-1, IL-6, IL-8, and IL-10 levels
Four milliliters of venous blood was collected from the ear marginal vein after fasting for 12 h, as well as at 2, 4, 6, and 8 h after FML in week 1 (W1), W4, W8, and W12, and then placed in a tube containing EDTA-anticoagulant. Samples were centrifuged for 15 min at 1000 xg using the automatic biochemical analyzer (Beckman, Brea, CA, USA) and the supernatant was stored in tubes at -80°C. After all experiments, an enzyme-linked immunosorbent assay (ELISA) was performed to detect the levels of plasma ET-1 (rabbit ET-1 detection kit, Shanghai Bogoo Biotechnology Ltd., Shanghai, China), IL-6 (rabbit IL-6 detection kit, Shanghai Bogoo Biotechnology Ltd.), IL-8 (rabbit IL-8 detection kit, Shanghai Westang Biotechnology Ltd., Shanghai, China), and IL-10 (rabbit IL-10 detection kit, Shanghai Westang Biotechnology Ltd.).
Ultrasound observation of abdominal aorta
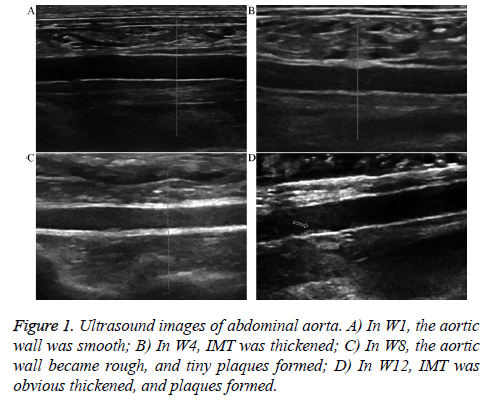
In W1, 4, 8, and 12, all animals were intramuscularly injected SumianxinII (0.1-0.2 mL/kg) for anesthesia, and then placed in the supine position to detect the cross-section and longitudinal section of the abdominal aorta from top to bottom along the abdominal midline (color Doppler ultrasound, MyLab 90, vascular probe frequency 7-10 MHz, ESAOTE, Genoa, Italy); conditions such as whether the vascular wall was smooth, the vascular cavity showed stenosis, the intimal-media thickness (IMT) was thickened, and plaques formed on the abdominal aortic wall, as well as their morphologies and sizes, were observed and recorded.
Pathological examination of abdominal aorta
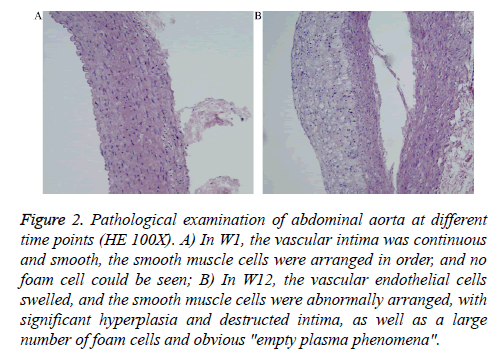
At the end of the experiment (W12), all animals were sacrificed and the abdominal aorta was isolated for conventional hematoxylin and eosin (H&E) staining followed by light microscopy (Olympus BX53, Tokyo, Japan) to observe the ATH conditions on the vascular cross-sections.
Statistical analysis
SPSS13.0 statistical software was used to analyze and process the experimental data (SPSS, Inc., Chicago, IL, USA). The measurement data were expressed as ͞x ± s and analysed using bi-time factor repeated measurement, with P<0.05 considered statistically significant.
Results
Changes in plasma ET-1, IL-6, IL-8, and IL-10 at different time points
The continuous high-fat diets and FML induced changes in endothelial function and inflammatory cytokines. As shown in Tables 1-4, compared with T0, ET-1 was increased in W4, W8, and W12 (P<0.05), IL-8 was increased in W4 (P<0.05) and IL-10 was increased in W4 and W8 (P<0.05). Compared with T0 in same weeks after administration of the FML, ET-1 was significantly increased at W0-T2, and W12-T1 (P<0.05), IL-8 was increased at W0-T1-3, W4-T1-2, and W8-T1-3 (P<0.05), and IL-10 was increased at W0-T1-4, W4-T2-4, and W12-T1-2 (P<0.05).
| Time | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| W1 | 102.86 ± 5.91 | 114.29 ± 18.15 | 128.89 ± 12.55* | 122.82 ± 18.79 | 121.67 ± 16.79 |
| W4 | 134.89 ± 11.74# | 142.82 ± 6.27 | 136.65 ± 5.38 | 137.59 ± 3.62 | 130.05 ± 15.07* |
| W8 | 129.19 ± 2.06# | 135.54 ± 15.27 | 134.95 ± 9.56 | 128.83 ± 9.23* | 119.84 ± 6.91* |
| W12 | 128.21 ± 1.61# | 146.20 ± 7.92* | 128.41 ± 11.56 | 120.55 ± 7.53 | 126.42 ± 8.21 |
Table 1. Changes of plasma ET-1 at different time points (x s, ng/L).
| Time | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| W1 | 96.84 ± 18.97 | 85.05 ± 12.58 | 98.95 ± 9.42 | 87.67 ± 25.19 | 94.61 ± 28.05 |
| W4 | 97.14 ± 20.85 | 93.96 ± 7.64 | 104.35 ± 8.17 | 107.73 ± 26.41 | 103.80 ± 27.41 |
| W8 | 89.22 ± 23.17 | 85.24 ± 23.61 | 71.59 ± 24.08* | 87.91 ± 23.43 | 90.20 ± 10.25 |
| W12 | 85.89 ± 23.91 | 67.43 ± 14.03 | 58.29 ± 11.73* | 71.01 ± 23.97 | 76.20 ± 27.23 |
Table 2. Changes of plasma IL-6 at different time points (x s, ng/L).
| Time | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| W1 | 68.34 ± 7.30 | 76.03 ± 8.56* | 84.45 ± 9.87* | 86.88 ± 7.00* | 68.20 ± 4.97 |
| W4 | 97.55 ± 5.45# | 101.25 ± 3.92* | 105.98 ± 5.87* | 103.94 ± 6.34 | 98.62 ± 6.49 |
| W8 | 74.04 ± 7.06 | 78.42 ± 9.84* | 82.66 ± 7.05* | 83.55 ± 10.81* | 75.53 ± 9.64 |
| W12 | 70.12 ± 7.29 | 69.79 ± 10.90 | 75.29 ± 10.29 | 74.83 ± 10.27 | 69.30 ± 9.58 |
Table 3. Changes of plasma IL-8 at different time points (x s, ng/L).
| Time | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| W1 | 108.73 ± 13.15 | 124.59 ± 18.65* | 138.79 ± 18.15* | 136.64 ± 14.64* | 121.36 ± 15.4* |
| W4 | 125.11 ± 16.36# | 133.21 ± 26.67 | 143.74 ± 19.21* | 139.92 ± 20.74* | 137.03 ± 18.61* |
| W8 | 120.39 ± 16.32# | 125.02 ± 15.81 | 125.82 ± 16.25 | 129.44 ± 6.27 | 115.86 ± 11.42 |
| W12 | 113.71 ± 13.04 | 122.27 ± 12.93* | 124.86 ± 11.52* | 121.06 ± 13.87 | 104.66 ± 13.08* |
Table 4. Changes of plasma IL-10 at different time points (xs, ng/L).
Ultrasound inspection of abdominal aorta
At the end of the experiment, the abdominal aortas of the 10 animals all showed different degrees of ATH. The ultrasound images are shown in Figure 1. In W1, all animals showed smooth rather than rough abdominal aortic walls and no IMT thickening; in W4, the abdominal aortic walls of all experimental animals were rough and showed different degrees of IMT thickening. In W8, 6 animals exhibited small plaques on the abdominal aortic wall and 4 animals exhibited obviously thickened IMT; in W12, all animals exhibited plaques on the abdominal aortic wall.
Pathological examination of abdominal aorta
At the end of the experiment, the abdominal aortas of the 10 animals showed ATH. The pathological results are shown in Figure 2. The vascular intima was continuous and smooth and the cells were arranged in order (Figure 2A). In W12, the vascular endothelial cells swelled and the smooth muscle cells were abnormally arranged, with a large number of foam cells (Figure 2B).
Figure 2: Pathological examination of abdominal aorta at different time points (HE 100X). A) In W1, the vascular intima was continuous and smooth, the smooth muscle cells were arranged in order, and no foam cell could be seen; B) In W12, the vascular endothelial cells swelled, and the smooth muscle cells were abnormally arranged, with significant hyperplasia and destructed intima, as well as a large number of foam cells and obvious "empty plasma phenomena".
Discussion
In this study, we investigated the dynamic changes in endothelial functions and inflammatory cytokines before and after FML in different development stages of ATH. The results showed that with prolonged high-fat feeding, plasma T0-ET-1 levels, plasma T0-IL-8 and plasma T0-IL-10 were significantly increased in W4, but plasma T0-IL-6 was not significantly increased in W4. And plasma T0-ET-1 levels then maintained at a high level, as well as plasma T0-IL-10 after W4, while the plasma T0-IL-8 then showed a decreasing trend. In addition, after the administration of FML every 4 weeks, ET-1 increased and then decreased, and was significantly increased at W1-T2, W4-T1, W8-T1, and W12-T1; IL-6 was slightly increased at W1-T2 and W4-T2-4, but significantly decreased at W8-T2 and W12-T2; IL-8 was significantly increased at W1-T1-3 and W8-T1-3, but decreased to the T0 level at T4, and its level was significantly increased at W4-T1-2 while not significantly changed in W12; IL-10 was increased at all postprandial time points in W1 and W4, which was much more obvious at T2 and T3, but the increase at W8-T2-3 was not significant, and the level was increased at W12-T1-3, but was significantly reduced at T4. Therefore, consistent with previous findings, continuous high-fat diets may induce endothelial dysfunction and inflammations, which can also significantly increase after a single FML. According to the results of this study, endothelial damage persisted throughout ATH progression. As lesion development progressed, injury time was continued from 4 h to 2 h after the meal; cell inflammatory cytokines and anti-inflammatory cytokines were mainly involved in the early development of ATH; in the late stage of ATH, inflammation was mild, but was most significant approximately 4 h after the high-cholesterol load.
Endothelial dysfunction is an initiating event towards the occurrence of ATH. ET-1 is a potent vasoconstrictor, damaging arterial endothelial functions and exhibiting proinflammatory effects [14], thus promoting the formation and development of ATH. Lerman et al. [15] found that patients in the early stage of ATH and coronary artery endothelial dysfunction exhibited increased blood ET-1 levels. Pan et al. [16] found that after feeding with high-fat and high-salt alcohol diet for 13 weeks, the serum ET-1 level in rats was significantly increased. Vogel et al. [17] found that at 4 h after a high-FML, healthy subjects exhibited seriously damaged endothelium-dependent vasodilation. The results of this study are consistent with those of previous studies, particularly that plasma T0-ET-1 was maintained at a high level during the various stages of ATH; further administration of FML significantly increased endothelial damage. As ATH progressed, the postprandial ET-1 peak time was advanced from 4 h to 2 h after the meal, indicating that patients with ATH always exhibited endothelial dysfunction and show greater effects from the FML. Therefore, these subjects should avoid high-FML. This may be related to the lower tolerance of sustained injury stimuli after endothelial function injury, as well as with the influence of a continuous high-fat diet on hepatic metabolic functions.
Inflammation is the basis for the occurrence and development of cardiovascular diseases, and excessive inflammation may induce and promote ATH and cause acute and chronic ischemic events [18]. IL-6 potently alters C-reactive protein-promoted ATH progress [19]. IL-8 activates neutrophils, thus causing the proliferation and aggregation of vascular smooth muscle cells and participating in the early formation of ATH [20]. Circulating IL-6 and IL-8 may promote inflammation and local angiogenesis and increase the risk of thrombosis, so they may be involved in acute and chronic vascular events in patients with coronary heart disease [21,22]. In this study, we found that during ATH development, the T0-IL-6 level in W4 was not significantly increased, but IL-8 was significantly increased, indicating that long-term high-fat diet-induced inflammation plays important roles in vascular remodeling and plaque formation in the early progressive stages of the disease. Thus, atherosclerotic diseases require early intervention and primary prevention measures in populations with a high risk of cardiovascular diseases.
Previous studies have shown that endothelial damage caused functional activation is related with cell inflammatory factors after a single high-FML. The IL-6 levels in patients with type 1 diabetes and healthy people were both significantly increased after high-fat diets [23]. The IL-6 level in abdominally obese women 1 h after high-fat meals was decreased, but significantly increased at 4 and 6 h after high-fat meals; however, the IL-6 level in slim females was significantly increased at 4 h after the meal [24]. Two hours after a saturated fatty acid meal, the IL-6 level in healthy mice was instantly and dramatically increased [25], and the IL-6 levels in healthy men at 4 and 6 h after a single high-fat meal were significantly increased [26,27]. The plasma IL-8 concentration in healthy men 2 h after a high-fat meal was not significantly increased [28], but increased significantly 4 h later [23]. In this study, the changes in IL-6 were slightly different from those reported in previous studies, the levels of which at W8-T2 and W12-T2 were reduced. The results for IL-8 were consistent with those of previous studies, which were increased at T2-3, significantly affected by FML when ATH existed or progressed, and induced the aggravation of inflammation. In contrast, the body is in the postprandial state in most of the time, so healthy people and patients with cardiovascular diseases all should pay attention to low postprandial inflammation to reduce the risk of cardiovascular diseases.
IL-10 may negatively regulate lipid deposition and inflammations and have anti-atherosclerotic effects [29]. Caligiuri et al. [30] found that the low-density lipoprotein levels and procoagulant activities in rats with insufficient apolipoprotein E and IL-10 were increased, and the instability of plaques was increased. Few studies have examined the changes and effects of high-fat diets on IL-10 in various stages of progression of ATH. The results of this study suggest that in the early progressive stage of ATH, the anti-inflammatory responses of the vascular walls were strong, and highcholesterol load may enhance these responses. These results indicate that IL-10, the in vivo ATH-protective factor, plays important roles in the early progressive stage of AS. With the aggravation of vascular wall lesions and the formation of plaques, the mechanisms of protective compensation were weakened. Significant anti-inflammatory responses can be induced 4-6 h after high-fat meals, corresponding to the time that worsened inflammation was observed.
This study was limited by the sample size, individual differences in the experimental animals, and the total experimental time, which may have influenced the results. The changes in inflammation during ATH require further investigation with increased sample sizes and extended experimental times. Limited by the different conditions in different experimental stages, ECG was only applied to observe the conditions of the abdominal aorta, and follow-up studies should observe other arteries such as the coronary artery or record changes in plaques in order to verify our results.
In summary, both long-term high-fat meals and a single highfat diet load can cause vascular endothelial injury and inflammation. Inflammation was severe during the early stage of ATH and high-fat meal 4-6 h after the load. And those research results are in line with previous related study. Nevertheless the mechanisms between high-fat diet load and inflammation and atherosclerosis are reported rarely. In general, healthy diets are particularly important for the health of the population or for those with progressive cardiovascular diseases. Unhealthy diets not only induce the occurrence and development of ATH, but also affect the stability of plaques. The 4th–6th-h after high-fat diets may be a high-risk period of vascular events, and thus particular attention should be paid to this period.
Acknowledgment
This work was funded by Shanxi Provincial Natural Science Foundation (2014011040-6).
References
- Ross R. Cell biology of atherosclerosis. Annu Rev Physiol 1995; 57: 791-804.
- STABILITY Investigators, White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, López-Sendón J, Manolis AJ, Mohler ER 3rd, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014; 370: 1702-1711.
- van Bussel BC, Soedamah-Muthu SS, Henry RM, Schalkwijk CG, Ferreira I, Chaturvedi N, Toeller M, Fuller JH, Stehouwer CD; EURODIAB Prospective Complications Study Group. Unhealthy dietary patterns associated with inflammation and endothelial dysfunction in type 1 diabetes: the EURODIAB study. Nutr Metab Cardiovasc Dis 2013; 23: 758-764.
- Teng KT, Chang CY, Chang LF, Nesaretnam K. Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutr J 2014; 13: 12.
- Patsch JR, Miesenböck G, Hopferwieser T, Mühlberger V, Knapp E, Dunn JK, Gotto AM Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb 1992; 12: 1336-1345.
- Giannattasio C, Zoppo A, Gentile G, Failla M, Capra A, Maggi FM, Catapano A, Mancia G. Acute Effect of High-Fat Meal on Endothelial Function in Moderately Dyslipidemic Subjects. Arterioscler Thromb Vasc Biol 2005; 25: 406-410.
- Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, Dandona P. Prolonged reactive oxygen species generation and nuclear factor-?B activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 2007; 92: 4476-4479.
- Hozumi T, Eisenberg M, Sugioka K, Kokkirala AR, Watanabe H, Teragaki M, Yoshikawa J, Homma S. Change in coronary flow reserve on transthoracic Doppler echocardiography after a single high-fat meal in young healthy men. Ann Intern Med 2002; 136: 523-528.
- van Bussel BC, Henry RM, Ferreira I, van Greevenbroek MM, van der Kallen CJ, Twisk JW, Feskens EJ, Schalkwijk CG, Stehouwer CD. A healthy diet is associated with less endothelial dysfunction and less low-grade inflammation over a 7-year period in adults at risk of cardiovascular disease. J Nutr. 2015; 145: 532-540.
- Barton M, Traupe T, Haudenschild CC. Endothelin, hypercholesterolemia and atherosclerosis. Coron Artery Dis 2003; 14: 477-490.
- Marino F, Tozzi M, Schembri L, Ferraro S, Tarallo A, Scanzano A, Legnaro M, Castelli P, Cosentino M. Production of IL-8, VEGF and Elastase by Circulating and Intraplaque Neutrophils in Patients with Carotid Atherosclerosis. PLoS One 2015; 10: e0124565.
- Wang JM, Sica A, Peri G, Walter S, Padura IM, Libby P, Ceska M, Lindley I, Colotta F, Mantovani A. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb 1991; 11: 1166-1174.
- Kolodgie FD, Katocs AS, Largis EE, Wrenn SM, Cornhill JF, Herderick EE, Lee SJ, Virmani R. Hypercholesterolemia in the rabbit induced by feeding graded amounts of low level cholesterol. Arteriosclerosis Thrombosis and Vascular Biology 1996; 16: 1454-64.
- Wang C, Liu J, Guo F, Ji Y, Liu N. Endothelin-1 induces the expression of C-reactive protein in rat vascular smooth muscle cells. Biochem Biophys Res Commun 2009; 389: 537-542.
- Lerman A, Holmes DR Jr, Bell MR, Garratt KN, Nishimura RA, Burnett JC Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation 1995; 92: 2426-2431.
- Pan DD, Gao JL, Chen SH, Tang QJ, Zhu EW, Lv GY. Effect of improper diets on blood viscosity in SD rats in high-salt and fat diet and alcohol abuse simulation model. Zhongguo Zhong Yao Za Zhi 2015; 40: 1560-1564.
- Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 1997; 79: 350-354.
- Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem 2007; 282: 18265-18275.
- Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 2002; 105: 1890-1896.
- Moreau M, Brocheriou I, Petit L, Ninio E, Chapman MJ, Rouis M. Interleukin-8 mediates downregulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: relevance to stability of atherosclerotic plaque. Circulation 1999; 99: 420-426.
- Mendall MA, Patel P, Asante M, Ballam L, Morris J, Strachan DP, Camm AJ, Northfield TC. Relation of serum cytokine concentration to cardiovascular risk factors and coronary heart disease. Heart 1997; 78: 273-277.
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 2009; 27: 165-197.
- Lassenius MI, Mäkinen VP, Fogarty CL, Peräneva L, Jauhiainen M, Pussinen PJ, Taskinen MR, Kirveskari J, Vaarala O, Nieminen JK, Hörkkö S8, Kangas AJ, Soininen P, Ala-Korpela M, Gordin D, Ahola AJ, Forsblom C, Groop PH, Lehto M. Patients with type 1 diabetes show signs of vascular dysfunction in response to multiple high-fat meals. Nutr Metab (Lond) 2014; 11: 28.
- Manning PJ, Sutherland WH, McGrath MM, de Jong SA, Walker RJ, Williams MJ. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity (Silver Spring). 2008; 16: 2046-2052.
- Magné J, Mariotti F, Fischer R, Mathé V, Tomé D, Huneau JF. Early postprandial low-grade inflammation after high-fat meal in healthy rats: possible involvement of visceral adipose tissue. J Nutr Biochem 2010; 21: 550-555.
- Brandauer J, Landers-Ramos RQ, Jenkins NT, Spangenburg EE, Hagberg JM, Prior SJ. Effects of prior acute exercise on circulating cytokine concentration responses to a high-fat meal. Physiol Rep 2013; 1: e00040.
- Schmid A, Petry N, Walther B, Bütikofer U, Luginbühl W, Gille D, Chollet M, McTernan PG, Gijs MA, Vionnet N, Pralong FP, Laederach K, Vergères G. Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br J Nutr 2015; 113: 1853-1861.
- van Oostrom AJ, Sijmonsma TP, Verseyden C, Jansen EH, de Koning EJ, Rabelink TJ, Castro Cabezas M. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J Lipid Res 2003; 44: 576-583.
- Rubic T, Lorenz RL. Downregulated CD36 and oxLDL uptake and stimulated ABCA1/G1 and cholesterol efflux as anti-atherosclerotic mechanisms of interleukin-10. Cardiovasc Res 2006; 69: 527-535.
- Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med 2003; 9: 10-17.