Case Report - Ophthalmology Case Reports (2022) Volume 6, Issue 1
Rehabilitation of a patient with anterior segment combined pathology
Shanturova M*Second Surgical Department of S.N. Fyodorov “Eye Microsurgery”, Federal State Institution, 664033, 337 Lermontov STR, Irkutsk, Russian Federation
- *Corresponding Author:
- Marina Shanturova
Second surgical department
664033, 337 Lermontov str
Irkutsk, Russian, Federation
Tel: +7 (952) 632-25-45
E-mail: shanturovam@mail.ru
Received: 21-Dec-2021, Manuscript No. OER-22-52588; Editor assigned: 23-Dec-2021, PreQC No. OER-22-52588 (PQ); Reviewed: 06-Jan-2022, QC No OER-22-52588; Revised: 10-Jan-2022, Manuscript No. OER-22-52588 (R); Published: 17-Jan-2022, DOI:10.35841/oer-6.1.102
Citation: Shanturova M. Rehabilitation of a patient with anterior segment combined pathology. Ophthalmol Case Rep. 2022;6(1):102
Abstract
Induced keratectasia after anterior keratotomy usually develops some years after the surgery and may cause unpredictable consequences. The healing of keratotomic incisions and changes in keratotopography caused by this process require studying. Here we present successful surgical treatment of a patient with bullous keratopathy, keratectasia associated with a previously performed tangential keratotomy, condition after phakic IOL implantation, incomplete complicated cataract and high myopia in the anamnesis. The patient underwent revision of the tangential keratotomic corneal incision, phacic IOL removal, and phacoemulsification with implantation of the capsular ring, Descemet stripping automated endothelial keratoplasty (DSAEK) with femtosecond laser assistance. Perioperative and postoperative periods were without complications. Complete corneal epithelization had been achieved by 6 –7 day. A year after the surgery, the cornea was transparent. The use of selective approaches in keratoplasty and microinvasive technologies for cataract removal allows successfully rehabilitating the patient with severe anterior segment combined pathology.
Keywords
Keratotomy, Keratectesia, Bullous keratopathy, DSAEK.
Introduction
The benefits of selective posterior corneal grafting in the treatment of endothelial dysfunction have recently been proven. Descemet stripping automated endothelial keratoplasty (DSAEK) significantly reduces the risk of perioperative and postoperative complications. It is typically performed simultaneously with cataract surgery. However, DSAEK is not indicated in cases with irreversible changes in the corneal stroma in the optical zone. Recently there have been a number of keratotomy patients who need the anterior segment surgery. And some of these patients have irregular scarring and induced corneal ectasia which is a severe keratotomy complication manifested by peripheral protrusion, corneal stroma thinning in the inferior segment, and partial wound dehiscences of one or more keratotomic scars [1-3]. Literature review has not shown any reports on DSAEK simultaneous performed with the anterior corneal stroma reconstruction in keratotomy patients.
We aimed at describing the first case of successful surgical treatment of a bullous keratopathy patient with induced keratectasia associated with previously performed tangential keratotomy, condition after phakic IOL implantation, cataracts, and high myopia in the anamnesis.
Case report
This clinical case was approved by the institutional review board of Irkutsk branch of S. N. Fyodorov “Eye microsurgery” Federal State Institution (Protocol No. 3, 27.02.2020). It conformed to the Declaration of Helsinki and a consent to publish an identifiable photograph was obtained from the patient.
In 2020 a 37-year-old woman was referred to our clinic with poor vision in eyes, watery eyes, a constant foreign body sensation, cloudy vision and photophobia in her right eye.
Medical history: high myopia, myopic astigmatism, amblyopia of both eyes; in 2007 posterior chamber phakic intraocular lens implantation (PCIOL) and tangential keratotomy of both eyes were performed; in 2019 the left eye twice received intravitreal administration of anti–vascular endothelial growth factor injections for myopic choroidal neovascularization.
The full ophthalmic examination revealed low visual acuity of the right eye (OD – 0.003; OS – 0.08 sph -1.0 cyl-1.0 ax 53 =0.2) and extremely high values of biometrics (OD – 31.33 mm, OS – 31.5 mm); intraocular pressure: OD – 20 mm Hg, OS – 19 mm Hg.
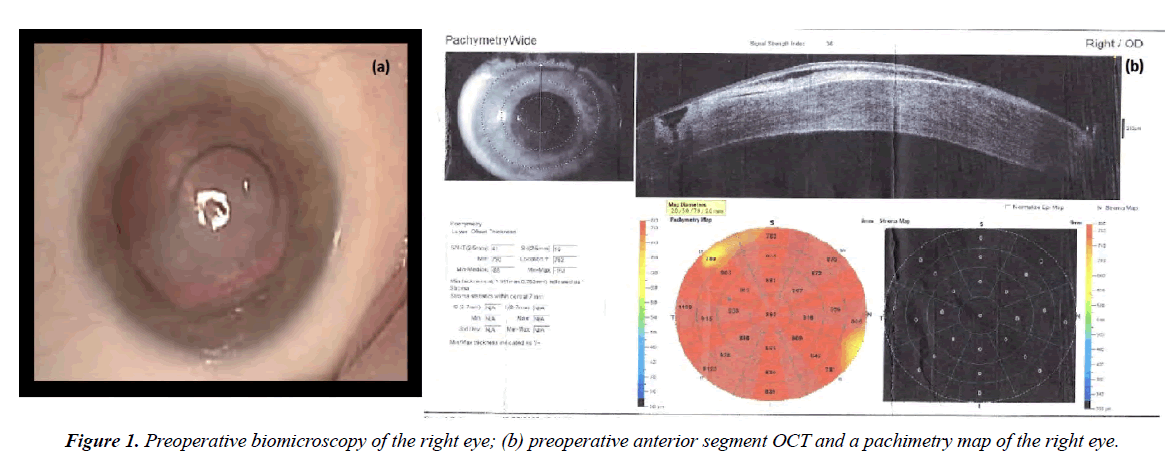
Biomicroscopic examination of the right eye revealed cloudy cornea, edematous, bullous epithelial changes. Keratectasia in the area of the tangential corneal incision. The position of the PCIOL is correct. The cortical lens layers are opacified. The fundus details are not ophthalmoscoped (Figure 1a). Anterior segment optical coherence tomography (AS-OCT) revealed pronounced corneal edema up to 1000 μm. The stromal profile is altered due to hypertrophied, partially detached epithelium. A gaping corneal stroma defect is determined in the projection of the tangential incision, almost up to the Descemet membrane, filled with a loose epithelial plug (Figure 1b).
Clinical findings were evaluated and bullous keratopathy, induced keratectasia, condition after PCIOL implantation and tangential keratotomy, incomplete complicated cataract, high myopia in the anamnesis of the right eye were diagnosed. The patient underwent surgical treatment of the right eye – tangential incision revision, removal of the PCIOL, phacoemulsification with the capsular tension ring implantation, DSAEK with a femtosecond laser assistance.
Surgical technique
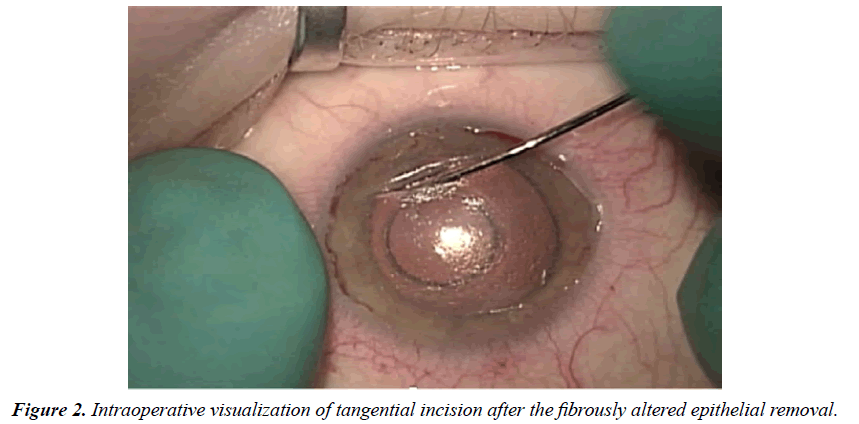
Significantly reduced corneal transparency can cause problems while performing phacoemulsification. However, after the removal of the fibrously altered epithelium layer, the anterior segment visualization improves. The gaping edges of tangential incision are clearly defined (Figure 2). At first using a femto spatula, we remove the epithelial plug from the tangential incision and refresh its edges. Gently delaminate the deep corneal layers by 1.0-1.5 mm on both sides of the tangential incision to improve the mobility of the incision edges. Then we apply a 10-00 continuous suture on the incision edges, thereby ensuring equal tension throughout its entire length.
All manipulations are carried out very carefully, so as not to perforate corneal deep layers and the Descemet membrane.
After that, we proceed to the next stage.
We perform 2 corneocentesis and the main 2.2 mm corneal incision. We introduce ophthalmic viscosurgical device (OVD) to maintain the depth of the anterior chamber. With two spatulas, we mobilize the phakic IOL and dislocate it into the anterior chamber. We fix the IOL with a micro-tweezer and cut in the optical part with scissors. After that, with two tweezers, we remove the IOL from the anterior chamber by rotating it. Next, we perform continuous circular anterior capsulorexis and routine phacoemulsification. Due to the anatomical eye parameters, there is no need for IOL implantation. However, to prevent the folding of the posterior lens capsule in the postoperative period, we implant the capsular tension ring in the capsular bag.
Then we mark the 8.0 mm descemetorexis zone. Using the reverse Sinskey hook, we perform descemetorexis according to the marking. This manipulation is significantly difficult to be performed in the projection of old corneal incisions (previous surgery). And especially big problems arise just in the zone of the tangential incision. However, using micro-tweezers, descemetorexis is successfully completed. After performing a basal iridectomy at 6 o'clock, we proceed to the donor material. We install the corneal-scleral disc in the artificial anterior chamber in an inverted way. A disk from the posterior layers of the cornea (endothelium, Descemet's membrane, stroma) with a diameter of 8 mm and a thickness of 150 μm is formed by a femtosecond laser in the donor cornea. Then, with a femto spatula, we separate the residual bridges of the corneal tissue and place the donor disk in the Busin glide. We expand the corneal incision to 4.5 mm. With a constant supply of irrigation solution, we implant the donor disk into the anterior chamber with a micro-tweezer. We fix the graft with aerocompression and apply stitches to the corneal incision and finally position the graft according to the marking.
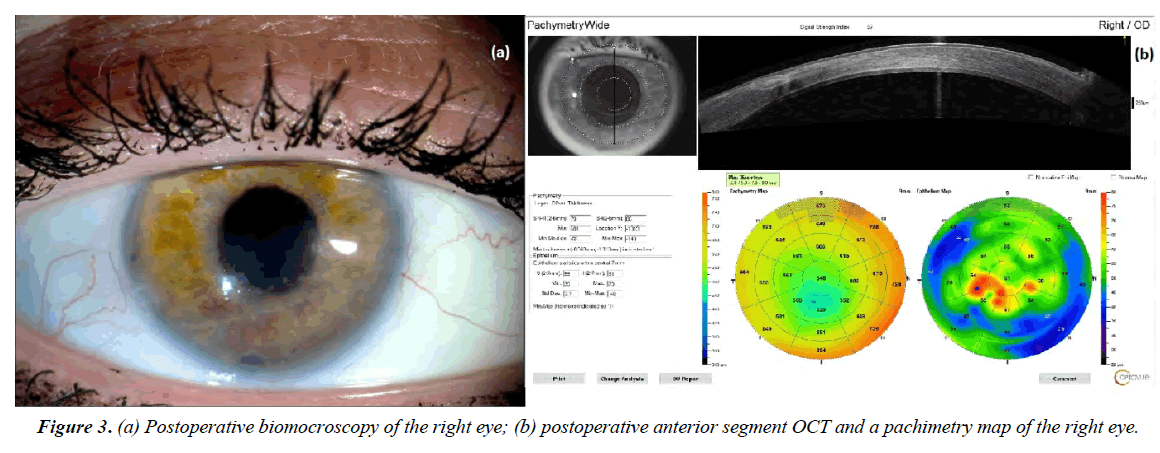
Postoperative period was without complications. Complete corneal epithelization was observed on the 6-7 days, but stromal edema persisted in the lower half of the cornea in the projection of manipulations with a tangential incision. Corneal sutures were removed 5 months after surgery. The cornea was transparent a year after the operation (Figure 3a). OCT of the anterior segment revealed the ideal graft position, complete normalization of the corneal thickness (central zone pachymetry - 540 μm) and a well-formed corneal scar (Figure 3b). Despite low visual acuity caused by macular degeneration, the patient does not complain and is satisfied with the results of the operation.
Discussion
The posterior chamber phakic IOL implantation is currently successfully used for the correction of high myopia. The method has both advantages and disadvantages. Refractive stability, preserved accommodation, reversibility and technical simplicity of the procedure are the benefits. But the method has got possible complications caused by the consequences of prolonged presence of PCIOL in the eye, including the effect on the corneal endothelium9-13. In uncomplicated postoperative course and a follow-up period from 1 to 5 years, the endothelial cells loss is on average from 1.8% to 12.3% [4], while in the phase of pronounced inflammatory response, the endothelial cells loss can gain about 66%.
In cases of ametropia and astigmatism combined, the implantation of PCIOL is supplemented by relaxing corneal incisions - tangential keratotomy. It should be noted that studies of the state of the cornea in the long-term period after keratotomy revealed low functional reserves of the corneal endothelium: decreased density, pleomorphism, and polymegathism of endothelial cells in 21.8% of cases [2,5]. Apparently, the use of two technologies that can potentially lead to a decrease in the density of endothelial cells in the long-term postoperative period, in this case caused a fatal loss of endothelium and the development of bullous keratopathy. In the presented clinical case we were interested in the unusual corneal condition 13 years after the tangential incision performed. This is just an example of induced keratectasia. The use of anterior keratotomy for the correction of myopia and myopic astigmatism at the end of the last century and the increasing number of repeated referrals of operated patients currently make the issue global that requires immediate attention. Induced keratectasia after anterior keratotomy usually develops 10-12 years after the surgery and may cause unpredictable consequences [2, 3, 6, 7]. The normal process of corneal wound healing involves regression of the epithelial plug within 6-14 days with the transformation of fibroblasts into fibrocytes and the corneal scar stabilization from 3 to 6 months after the surgery. In cases of radial and tangential keratotomy, delayed wound healing caused by epithelial plugs persisting up to 47 months after the surgery [8, 9].
The process of stromal corneal wound formation and remodeling is turned out to continue for many years after radial keratotomy, and after its completion, there is no continuity restoration between the collagen fibrils, but the creation of a stromal scar matrix. Moreover, the scar tissue is characterized by a weakly expressed cellular-fibrous matrix and the absence of proteoglycans [2,10]. An extremely sharp diamond knife is supposed to reduce operational stress, trauma and significantly slow down the process of fibroblast activation and corneal scar formation. Thus, despite of the time after radial keratotomy, the healing of keratotomic incisions occurs not as a slow process, but as an incomplete scarring process influenced by various factors [4, 10].
The healing of keratotomic incisions and changes in keratotopography caused by this process require further study. Especially in recent years, a sufficient number of patients with a keratotomy medical history need anterior segment surgery. Nevertheless, the use of selective approaches in keratoplasty and microinvasive technologies for cataract removal can successfully rehabilitate patients even in such difficult cases.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
References
- Seitz B, Langenbucher A, Hofmann-Rummelt C, et al. Nonmechanical posterior lamellar keratoplasty using the femtosecond laser (femto-plak) for corneal endothelial decompensation. Am J Ophthalmol. 2003; 136(4): 769–772.
- Pasikova NV. Long Term Follow-up of the Corneal State after Anterior Radial Keratotomy. Ophthalmology in Russia. 2018; 15(1): 38–42.
- Wellish KL, Glasgow BJ, Beltran F, et al. Corneal ectasia as a complication of repeated keratotomy surgery. J Refract Corneal Surg. 1994; 10(3): 360–364.
- Edelhauser HF, Sanders DR, Azar R, et al. Corneal endothelial assessment after ICL implantation. J Cataract Refract Surg. 2004; 30(3): 576–583.
- Mac Rae SM, Matsuda M, Rich LF. The effect of radial keratotomy on the corneal endothelium. Am J Ophthalmol. 1985; 100(4): 538–542.
- Shaikh S, Shaikh NM, Manche E. Iatrogenic keratoconus as a complication of radial keratotomy. J Cataract Refract Surg. 2002; 28(3): 553–555.
- Sharma N, Sachdev R, Jindal A, et al. Acute hydrops in keratectasia after radial keratotomy. Eye Contact Lens. 2010; 36(3): 185–187.
- Deg JK, Zavala EY, Binder PS. Delayed corneal wound healing following radial keratotomy. Ophthalmology. 1985; 92(6): 734–740.
- Binder PS, Waring GO 3rd, Arrowsmith PN, et al. Histopathology of traumatic corneal rupture after radial keratotomy. Arch Ophthalmol. 1988; 106(11): 1584–1590.
- Stainer GA, Shaw EL, Binder PS, et al. Histopathology of a case of radial keratotomy. Arch Ophthalmol. 1982; 100(9): 1473–1477
Indexed at, GoogleScholar, Cross Ref
Indexed at, GoogleScholar, Cross Ref
Indexed at, GoogleScholar, Cross Ref
Indexed at, GoogleScholar, Cross Ref
Indexed at, GoogleScholar, Cross Ref
Indexed at, GoogleScholar, Cross Ref
Indexed at, GoogleScholar, Cross Ref
Indexed at, GoogleScholar, Cross Ref