Research Article - Biomedical Research (2017) Volume 28, Issue 10
Regenerative property of PRF used as capping material in pulpotomy in dogs
Masoumeh Hassani Tabatabayi1, Azin Tavakoli2 and Bahareh Aghamohammadi Ameghani3*
1Department of Dental School, Tehran University of Medical Sciences, Tehran, Iran
2Department of Surgical Veterinary, Islamic Azad University, Garmsar Branch, Garmsar, Iran
3Department of Dental School, Tehran University of Medical Sciences, Tehran, Iran
- *Corresponding Author:
- Bahareh Aghamohammadi Ameghani
Department of Dental School
Tehran University of Medical Sciences, Iran
Accepted on March 06, 2017
Abstract
Among the new approaches to tissue regeneration platelet rich fibrin (PRF) is shown to have significant effect in different surgical fields like pulpotomy. The PRF clot forms a strong natural fibrin matrix with platelets and growth factors and makes a complex structure as a healing matrix. The blood of adult healthy dogs weighting 18 ± 3 kg centrifuged for 12 min at 10000 /rpm. The PRF was isolated. A cervical cavity was prepared in the three incisors of both upper and lower jaw. When the pink color of pulp was seen through the thin layer of dentin, a No.2 explorer was used to expose the pulp. In group A no treatment was performed and the cavities were filled only by zonalin cement. In group B, PRF was placed on exposure site and zonalin cement over it. All teeth were extracted and sections were viewed under light microscope. Different degrees of inflammation and necrosis were observed in pulpal tissues in group A. Dentinal bridge was not observed in group A. Different degrees of dentin bridge formation were observed in group B. Mean necrosis in group A (1) was significantly higher when compared to the mean degree of necrosis in group B (0.16). (P=0.001). Dentin bridge formation occurred in the pulpal tissue of the dog’s incisor teeth capped with PRF, which shows promising effects in healing of the wounded pulp.
Keywords
Dental pulp capping, Dentinogenesis, Necrosis, Platelet rich fibrin.
Introduction
The vitality of the pulp is very important to the tooth health and plays a significant role during decision making to manage pulpal pathologies. Pulpal viability is not only critical in the treatment of pulpal exposure in permanent teeth of the young patients subsequent to caries, but also in primary molar teeth [1]. Pulpal exposure may occur following dental caries, traumas and malformation of the teeth [2]. It has been proven that pulpotomy is effective treatment for preserving pulpal viability after pulpal exposure and in the presence of inflammation. The vitality of the pulp is maintained by the healing potential of the remaining radicular tissue and the biocompatibility of pulpotomy agents as well [3]. Therefore, pulpotomy is effective in treatment of reversible pulpal injuries through sealing of the pulp. Subsequently the tertiary dentine formation occurs [2]. Calcium hydroxide (Ca(OH)2) and Ca(OH) compounds has been used since 1920 and currently are considered as the gold standards of pulp capping [3-6]. However, many studies have been performed to introduce alternative biocompatible pulp capping materials. Among them Mineral trioxide aggregate (MTA) has been shown to be the material of choice for pulpotomy procedures. Many animal study results indicated the superiority of MTA to Ca(OH) when used as a direct pulp capping agent [7-9].
Among the newly introduced approaches to tissue regeneration platelet rich plasma (PRP) and platelet rich fibrin (PRF) are shown to have significant effect in healing in different surgical fields such as head and neck surgery, orthopedic surgery, cardiovascular surgery, and maxillofacial surgery [10,11]. PRP and PRF are non-expensive, easy to be prepared, and produce less inflammation during healing. A natural blood clot contains mainly red blood cells, approximately 5% platelets and less than 1% white blood cells (WBC). PRP is derived from the centrifugation of autologous blood. In order to be prepared in a gel formulation, PRP is mixed with thrombin and calcium chloride. Therefore, a high concentration of platelets, the associated growth factors and fibrinogen are included in PRP gel. The important difference in composition between PRP and PRF is the presence of a high concentration of platelets and native concentration of fibrinogen in PRP [4]. Preparation of PRF does not require thrombin and calcium chloride for activation and the process is even easier and less time consuming than preparation of PRP gel. Thus, the preparation of PRF is strictly autologous. PRF was first described by Choukroun et al. [12] and it is called the second generation platelet concentrate. It has several advantages over traditionally prepared PRP [12].
The PRF clot forms a strong natural fibrin matrix, which has almost all the platelets and growth factors of the blood harvest [2,3] and shows a complex structure as a healing matrix, including mechanical properties no other platelet concentrate offers. PRF can be considered as a natural fibrin-based biomaterial favorable to the development of a microvascularization and able to guide cell migration into wound area. In the study of Pathak et al. use of the PRF in a carious pulp exposure of a young permanent tooth with pulpitis made a successful result over 6 months [13]. In the study of Kumar et al. he used the PRF as pulp capping material over a permanent tooth of a 21 years old male and after 6 months the patient had no pain or radiograph changes [14].
Pulp cells residing in pulp clinically diagnosed with pulpitis might still have stem cell potential similar to healthy pulp cells and therefore might be a resource for autologous pulp regeneration which suggest opportunities for biologically based therapeutic approaches to dental tissue repair as well as providing valuable insights into how natural regenerative processes like PRP or PRF may be operating in the tooth [4]. Therefore, we designed a study to investigate the healing effects of PRF following vital pulp therapy in the incisor teeth of the dogs.
Materials and Methods
After approval was received from the University Research Committee, Azad University of Garmsar the procedure was started according to animal ethics guidelines. 10 ml blood sample of jugular vein was taken. Adult healthy male mixed breed dogs weighing 18 ± 3 kg were entered the study. The food was restricted for 8 h in all subjects prior to surgery. The blood collected in a tube without anticoagulant agents like EDTA. After one minute of resting, the tube centrifuged for 12 minutes at 10000 rpm. The white jelly part above the clot (Figure 1), which is PRF, was isolated. Then, general anesthesia performed using Acepromazine (0.01 mg/kg) as premedication and the combination of ketamine and diazepam (IV, 8.5 mg/kg and 0.2 mg/kg) for induction of anesthesia. The anesthesia maintained by inhalation of isoflurane in Oxygen following intubation. The oral chlorhexidine solution 1.2% was used for aseptic preparation of the oral cavity.
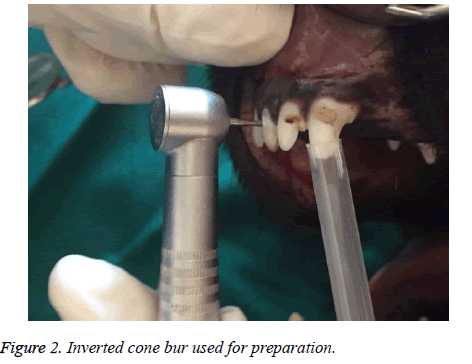
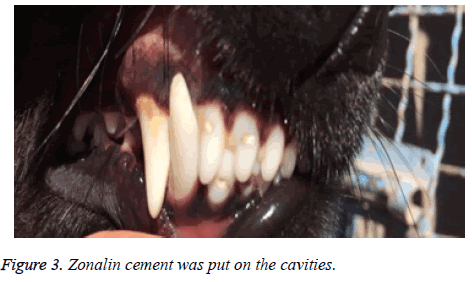
After anaesthetizing the dog and infiltration of one carpule of lidocaine with epinephrine, a cervical cavity with an inverted bur was made in the three incisors of both upper and lower jaw (Figure 2). When the pink color of pulp was seen through the thin layer of dentin, teeth were isolated with cotton rollsand, a No.2 explorer was used to expose the pulp. Cavities were washed with normal saline. A total of 20 incisor teeth were prepared in this way. After preparation of the cavity the teeth were assigned into two groups. In group A no treatment was performed and the cavities were filled by only zonalin cement. In group B, a 1 × 2 mm of PRF was brought to the exposure sites and placed over it and a zonalin cement covered the whole cavity (Figure 3).
The teeth were checked weekly for any signs of discoloration and inflammation. After two months the teeth, all teeth in both groups were extracted under general anesthesia. The teeth were fixed in 10% buffered formalin and processed in a tissue processor after decalcification was performed. Paraffinembedded tissue sections were stained by Harris hematoxylin and eosin (H&E) method. The stained sections were viewed under light microscope at 4X and 10X magnifications for examination. A single veterinary pathologist viewed the slides and graded them according to degree of inflammation, formation of hard tissues and dentinal bridge and necrosis. Inflammation was graded as non-existent (0), mild infiltration of inflammatory cells, Neutrophils and Leukocytes (1), moderate infiltration of inflammatory cells, Neutrophils and Leukocytes (2) and severe infiltration of inflammatory cells, Neutrophils and Leukocytes so that more than 2/3rd of the pulpal canal is involved (3).
Formation of dentinal bridge was graded as non-existent of dentinal bridge (0), mild sedimentation of hard tissues underneath and surround the used regenerative material (1), moderate sedimentation of hard tissues and thin layer of dentin bridge underneath and surround the pulp capping material (2) and full formation of dentinal bridge under the exposure area (3). Necrosis was graded as no necrosis (0) and existence of denatured proteins and autolysis in pulp tissue (1). Means of the measured variables were compared between the groups using ANOVA. P values less than 0.05 were considered statistically significant.
Results
Results of this study are illustrated according to histopathological parameters including degree of inflammation, formation of dentinal bridge and necrosis were used in this study to evaluate the regenerative property of PRF.
Inflammation degree
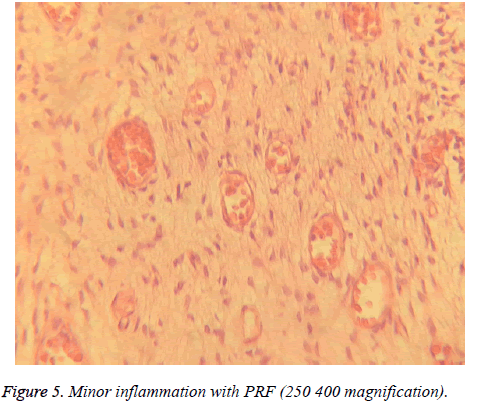
Different degrees of inflammation, from mild to even severe degrees were observed in pulpal tissues in groups A and B (Figures 4-6). However, results indicated that mean degree of inflammation was significantly higher in group A (2.25) in comparison to group B (0.75) (P=0.00). Results are illustrated in Table 1.
| Group A | Group B | |
|---|---|---|
| Inflammation degree | 2.25 | 0.75 |
| Dentinal bridge | 0 | 1.67 |
| Necrosis | 1 | 0.16 |
Table 1. Mean degree of inflammation, dentinal bridge and necrosis in group A and B.
Dentin bridge formation
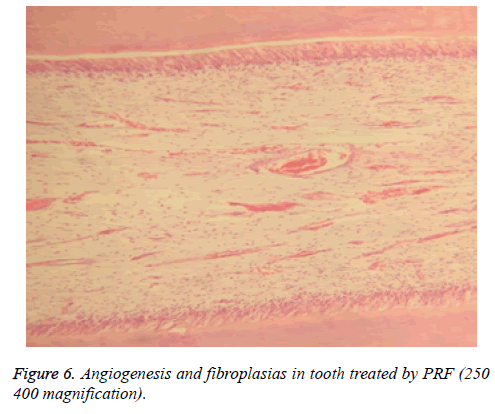
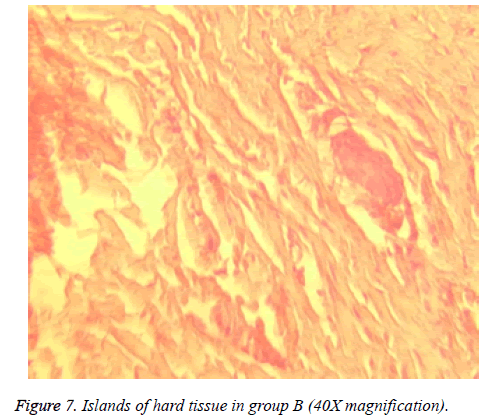
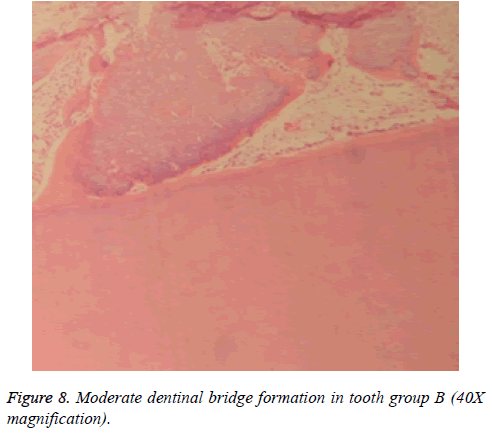
Dentinal bridge was not observed in group A. However, different degrees of dentin bridge formation were observed in group B (1.67) (Figures 7 and 8). Results are illustrated in Tables 1 and 2.
| Samples 1 and 2 | Test Statistical | Std. Error | Std.Test Statistical | Sig. | Adj.Sig. |
|---|---|---|---|---|---|
| Control-PRF | -18.000 | 4.000 | -4.500 | .000 | .000 |
Table 2. Each row tests the null hypothesis that the Sample 1 and Sample 2 distributions are the same. The significance level is 0.05.
Necrosis
Necrosis in the subjects in group A was obvious , and was significantly higher when compared to the necrosis in group B (P=0.001) Necrosis was observed in all pulpal tissues in group A, however, it was found in 2 pulpal tissues in group B as well.
Discussion
Pulpotomy is an accepted treatment for teeth with pulpal exposure and it is reported that the success rate of endodontic treatment is lower than that of initial treatment [13-15]. Since the preservation of the dental pulp is essential to maintain the teeth, attempts have been done to preserve the vital pulp and/or facilitate regeneration of the pulpal tissue. Thus, different materials and biomaterials have been examined for pulpotomy and pulp capping. Pulpotomy considered being successful when apoptosis of odontobalsts and pulpal cells occuring initially and finally reparative dentinogenesis takes place [16-18]. The purpose of this study was to evaluate the regenerative property of PRF as a pulp capping material in pulpotomy of the incisor teeth in dogs.
Different histological parameters regarding pulpal wound healing following pulp capping with PRF were recorded in this study, including degree of inflammation of the pulpal tissue, formation of Dentin Bridge and existence of necrosis. Results of the present study showed that inflammation was occurred in the both groups of the study. However, the inflammation was more evident in group A, that no treatment was applied. PRF produced mild to moderate inflammation in the subjects of group B. PRF includes dense fibrin network with leukocytes, cytokines, glycoproteins and growth factors. Basically leukocytes present in PRF have a key role in releasing of growth factors and anti-infectious activity. This explains, the relative existence of moderate inflammation of the pulpal tissue following pulp capping using PRF.
Dentin bridge was not formed in any of the subjects of group A due to severe inflammation. In contrast the bridge and reparatory dentine were observed in all of the subjects of group B. It is shown that the associated growth factors with PRF are released in the tissue during 1 to 4 weeks after applying of the PRF [19,20]. So as we expected, the regenerative property for PRF when used as pulp capping in group B was observed. Also the expected time for pulpal repair is during the first 3 weeks [21]. which was in agreement with result of the this study. Therefore, the observation of reparative dentin in the pulpal tissue is an optimistic sign of pulpal regeneration that was occurred in group B following pulpotomy. Huang et al. in 2010 reported that PRF causes proliferation of human dental pulp cells and increase the protein expression of osteoprotegerin and alkaline phosphatase activity. Therefore, in the presence of low amount of vital pulpal cells, odontoblast like cells will be produced and pulp-dentin complex will form [19].
The effectiveness of PRF in accelerating wound healing of different tissues have been studied and proved previously [18-30]. Growth factors are critical in signaling the formation and repair processes in dentine-pulp complex and they signal many of the important events in tooth morphogenesis and differentiation, also recapitulation of these processes may lead to tissue regeneration [31]. In addition microvascularization develops in the fibrin network of PRF promotes cell migration [24]. In current case an effort was made to use such growth factors in order to help healing the pulp after exposure . Animal incisors were used in many DPC studies [32-34]. For cavity preparation only class V cavities were prepared in this study, as the procedure was easy and functional loads during biting could be avoided [35-37]. Furthermore, our findings were similar with the results of Wang et al. data that have been revealed the pulp cells residing in pulp might have potential of stem cells and help the pulp for regeneration [38]. We obtained similar results in the present study.
As we expected the pulp with only zonalin coverage cavities led to necrosis in group A. However, necrosis occurred in 2 of the pulpal tissues in group B. This probably occurs due to organization of the inflammation and subsequent infection of the pulpal tissue. If the inflammation existed in the pulpal canal conquers the infection, regeneration will occur subsequently. As discussed earlier PRF was prepared with the dog's own blood and was placed in the exposure site of the teeth. The process of preparation of PRF is very easy, quick and unlike PRP activation with bovine’s thrombin is not needed. Also PRF is considered a potent autologous material, therefore, the risk of transported infected and blood borne diseases are reduced when PRF is used compared to other allograft, xenografts and biomaterials that are used as pulp capping agents [24,39,40].
Conclusion
As mentioned above, the formation of dentin bridge is essential in maintaining the viability of the pulp. The reparative dentin is formed in residual dental pulp. Therefore, in the absence of bleeding, PRF supplies growth factors and potential network for regeneration of the pulp [23]. It is concluded that mild to moderate inflammation and dentin bridge formation occurred in the pulpal tissue of the dog’s incisor teeth capped with PRF, which shows promising effects in healing of the wounded pulp. Also necrosis reported in 2 subjects of the groups treated with pulpotomy. These findings suggest biologically based treatment approaches to dental tissue healing and provide an insight how natural regenerative processes can be done in teeth. Further research on this subject is required with regard to operate on human teeth and more follow up time.
References
- Zander HA. Reaction of the pulp to calcium hydroxide. J Dental Res 1939; 18: 373-379.
- Moghaddame-Jafari S, Mantellini MG, Botero TM, McDonald NJ, Nör JE. Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J Endod 2005; 31: 387-391.
- Takita T, Hayashi M, Takeichi O, Ogiso B, Suzuki N, Otsuka K, Ito K. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endodont J 2006; 39: 415-422.
- Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Materials Res 1997; 37: 432-439.
- Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod 1995; 21: 349-353.
- Kiran N, Mukunda K, Tilak Raj T. Platelet Concentrates: A promising innovation in dentistry. J Dental Sci Res 2011; 2: 50-61.
- Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxill Surgery 1997; 55: 1294-1299.
- Kiran N, Mukunda K, Tilak Raj T. Platelet Concentrates: A promising innovation in dentistry. J Dental Sci Res 2011; 2: 50-61.
- Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res 2008; 19: 42-46.
- Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing 2013; 10: 23.
- Carlson NE, Roach RB Jr. Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc 2002; 133: 1383-1386.
- Kalaskar RR, Damle SG. Comparative evaluation of lyophilized freeze dried platelet derived preparation with calcium hydroxide as pulpotomy agents in primary molars. J Indian Soc Pedodont Prevent Dentistry 2004; 22: 24-29.
- Pathak S, Bansode P, Ahire C. PRF as a pulpotomy medicament in a permanent molar with pulpitis: a case report. IOSR J Dental Med Sci 2014; 1: 5-9.
- Kumar Das U, Mitra A, Bose N. The healing touch of PRF:a case report. Int J Healthcare Biomed Res 2014; 2: 37-41.
- Imura N, Pinheiro ET, Gomes BP, Zaia AA, Ferraz CC, Souza-Filho FJ. The outcome of endodontic treatment: a retrospective study of 2000 cases performed by a specialist. J Endod 2007; 33: 1278-1282.
- Kitamura C, Ogawa Y, Nishihara T, Morotomi T, Terashita M. Transient co-localization of c-Jun N-terminal kinase and c-Jun with heat shock protein 70 in pulp cells during apoptosis. J Dental Res 2003; 82: 91-95.
- Kitamura C, Nishihara T, Ueno Y, Nagayoshi M, Kasugai S, Terashita M. Thermotolerance of pulp cells and phagocytosis of apoptotic pulp cells by surviving pulp cells following heat stress. J Cell Biochem 2005; 94: 826-834.
- Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol 2011; 38: 925-932.
- Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod 2010; 36: 1628-1632.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surgery Oral Med Oral Pathol Oral Radiol Endod 2006; 101: e37-44.
- Shivashankar VY, Johns DA, Vidyanath S, Kumar MR. Platelet Rich Fibrin in the revitalization of tooth with necrotic pulp and open apex. J Conserv Dent 2012; 15: 395-398.
- Sopariwala N, Garg S. Regenerative Endodontic Procedure using Platelet-Rich Fibrin to Treat Traumatized Immature Permanent Tooth: a Case Report. IOSR J Dental Med Sci 2015; 5: 36-40.
- Kitamura C, Nishihara T, Terashita M, Tabata Y, Jimi E, Washio A, Hirata S. Regeneration approaches for dental pulp and periapical tissues with growth factors, biomaterials, and laser irradiation. Polymers 2011; 3: 1776-1793.
- Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen 2008; 16: 356-363.
- Amir FA, Gutmann JL, Witherspoon DE. Calcific metamorphosis: a challenge in endodontic diagnosis and treatment. Quintessence Int 2001; 32: 447-455.
- Asgary S, Eghbal MJ. The effect of pulpotomy using a calcium-enriched mixture cement versus one-visit root canal therapy on postoperative pain relief in irreversible pulpitis: a randomized clinical trial. Odontology 2010; 98: 126-33.
- Aqrabawi J. Endodontics: Sealing ability of amalgam, super EBA cement, and MTA when used as retrograde filling materials. Br Dental J 2000; 188: 266-268.
- Haglund R, He J, Jarvis J, Safavi KE, Spångberg LS, Zhu Q. Effects of root-end filling materials on fibroblasts and macrophages in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95: 739-745.
- Balto HA. Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: a scanning electron microscope study. J Endodontics 2004; 30: 25-29.
- Asgary S, Eghbal MJ. Treatment outcomes of pulpotomy in permanent molars with irreversible pulpitis using biomaterials: a multi-center randomized controlled trial. Acta Odontologica Scandinavica 2013; 71: 130-136.
- Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res 2008; 19: 42-46.
- Smith AJ. Vitality of the dentin-pulp complex in health and disease: growth factors as key mediators. J Dent Educ 2003; 67: 678-689.
- Obersztyn A. A new method for testing drugs used in direct pulp capping on a rat incisor as an experimental model. Czas Stomatol 1965; 18: 213-220.
- Hu JC, Zhang C, Yun SS, Qian Q, Ranly DM. Platelet-derived growth factor-BB and epidermal growth factor as pulp capping medicaments in rat incisors. J Hard Tissue Biol 1997; 6: 121-129.
- Sloan AJ, Smith AJ. Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-beta isoforms 1-3 in vitro. Arch Oral Biol 1999; 44: 149-156.
- Nakamura Y, Hammarström L, Lundberg E, Ekdahl H, Matsumoto K, Gestrelius S, Lyngstadaas SP. Enamel matrix derivative promotes reparative processes in the dental pulp. Adv Dental Res 2001; 15: 105-107.
- Bidar M, Gharechahi M, Shahrami F, Safari MK, Forghani M. An in Vitro Evaluation of Coronal Microleakage Through Four Temporary Restorations by Dye Penetration. Jundishapur Sci Med J 2011; 6: 605-613.
- Wang Z, Pan J, Wright JT, Bencharit S, Zhang S, Everett ET, Teixeira FB, Preisser JS. Putative stem cells in human dental pulp with irreversible pulpitis: an exploratory study. J Endod 2010; 36: 820-825.
- Kiran N, Mukunda K, Tilak Raj T. Platelet Concentrates: A promising innovation in dentistry. J Dental Sci Res 2011; 2: 50-61.
- Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod 2005; 31: 711-718.