Research Article - Current Pediatric Research (2018) Volume 22, Issue 2
Recall rate and incidence of congenital hypothyroidism: A 5-year experience of hospital-based study in Bahrain
Eman Shajira, Babiker Hassan, Fahad Al-Qashar, Haya AlkhayyatDepartment of Pediatrics, Bahrain Defence Force Hospital, Royal Medical Services, Riffa, Kingdom of Bahrain.
- *Corresponding Author:
- Eman Shajira
Department of Pediatrics, Bahrain Defense Force Hospital
Royal Medical Services, Riffa, Bahrain.
Tel: +97317766959
E-mail: eman.shajera@bdfmedical.org
Accepted date: April 25, 2018
Abstract
Background: Different neonatal screening programs for congenital hypothyroidism (CH) have been implemented to prevent its devastating effects on mental development. High recall rate and false positive results subject families to undue stress and impose financial burdens on health care expenditures. Objective: to investigate the CH recall rate and establish an optimum cord blood thyroid-stimulating hormone (CBTSH) level for CH diagnosis. Methods: retrospective cross-sectional study was conducted in Bahrain Defense Force hospital from Jan 2012 to Dec 2016. CBTSH and back-up free thyroxine (T4) results from all newborn infants born during this period were analyzed. All of the infants with CBTSH >37.5 μIU/ml were recalled for confirmatory tests. Results: Out of 20,885 newborns, 413 infants were recalled (recall rate 1.98%). CH incidence was 1:1489, mean CBTSH was 99.5 μIU/ml (95% confidence interval (CI) 98.7-100.3 μIU/ ml), and mean free T4 was 9.1 pmol/l (95% CI, 6.64-11.57 pmol/l). A receiver operating characteristic (ROC) curve identified the new CBTSH cutoff value as 93 μIU/ml. Conclusion: Our study supports setting a higher CBTSH cutoff value based on our population findings and recommends both CBTSH and simultaneous free cord T4 measurements as the ideal screening approach.
Keywords
Infants, Health care, Stress.
Abbreviations
CH: Congenital Hypothyroidism; CBTSH: Cord Blood Thyrotropin Stimulating Hormone; ROC: Receiver-Operating Characteristic
Introduction
Congenital hypothyroidism (CH) is one of the most common preventable causes of intellectual disability. It affects 1:2000 to 1:4000 new-born infants worldwide [1,2]. The clinical manifestations are usually subtle or not present at birth [3]. Different neonatal screening programs for CH have been implemented in both developed and some developing countries. It is estimated that only 1/4 of the worldwide birth population undergo screening for CH [4,5].
In many countries, different mass screening programs are performed for early diagnosis and treatment of CH [6], yet most have encountered problems such as high recall rate and therefore, more substantial numbers of falsepositive results are found, which carry a risk for unjustified anxiety and psychological harm in families. On the other hand, fewer false positive results increase physicians’ and parents’ trust, reduces families’ psychological stress, efficiently organize the medical staff members’ workload and reduce expenses by minimizing unnecessarily repeated laboratory tests [7,8]. In this study, we determined both CH incidence and recall rates based on our local neonatal population that was screened over the study period and redefined the cord blood thyrotropin-stimulating hormone (CBTSH) cut-off levels for identifying neonates at risk of CH.
Materials and Methods
Materials and Subjects
This is a retrospective cross-sectional study that was carried out from January 2012 to December 2016 in Bahrain Defense Force Hospital. All inborn deliveries conducted during this period were part of the study. Primary CBTSH with backup T4 measurements were assayed on single specimens using the electrochemiluminescence immunoassay “ECLIA” by Cobase immunoassay analyzer (ROCHE Diagnostics, Germany) with a measurement range of 0.005 to 100 μIU/ml and 0.3 to 100 pmol/l for TSH and free T4, respectively. For confirmation, infants were recalled if cord blood TSH was >37.5 μIU/ml, and serum TSH and free T4 measurements were obtained at an average of five days after birth. Thyroid scans using Tc- 99m were requested to determine the underlying etiology. Clinical data were obtained for all positive cases included sex, gestational age, nationality, type of delivery and single or multiple gestations.
Statistical Methods
Statistical data were analyzed using SPSS software version 19.0. Descriptive statistics were performed using the calculation of frequency, percentages, mean and standard deviation (SD). Sample characteristics were summarized using mean ± SD for numerical data and frequency and percentages for categorical data. The CBTSH values of all confirmed cases of CH and false positive cases were pooled to generate a receiver operating characteristic (ROC) curve using MedCalc statistical software to determine appropriate CBTSH cutoff value for CH diagnosis. The hospital ethics committee approval was obtained.
Results
The total number of babies born at Bahrain Defense Force Hospital during the study period was 20,855 newborn infants, of which 413 (1.98%) were identified to be at risk (Table 1) as they had CBTSH >37.5 μIU/ml. Out of that 78.5% were Bahraini and 237 infants (57.7%) were males. Term, pre-term, post-term and extremely pre-term infants accounted for 74.1%, 24.7%, 0.7% and 0.5%, respectively. Cesarean section was the mode of delivery of 57.1% infants compared to 36.6% spontaneous vaginal delivery (SVD) and 6.3% assisted vaginal delivery. (5.3%) were part of multiple gestations. In populations found to be at risk, mean CBTSH and T4 were 55.70 ± 18.53 μIU/mL and 23.51 ± 9.79 pmol/L, respectively (Figure 1).
| Variable | Frequency | Percentage | Range |
|---|---|---|---|
| Gestational Age | |||
| Preterm | 102 | 24.70% | - |
| Post-term | 3 | 0.70% | - |
| Extreme Preterm | 2 | 0.50% | - |
| Term | 306 | 74.10% | - |
| Gender | |||
| Male | 237 | 57.60% | - |
| Female | 176 | 42.60% | - |
| Nationality | |||
| Bahraini | 324 | 78.50% | - |
| Non-Bahraini | 89 | 21.50% | - |
| Delivery type | |||
| SVD | 151 | 36.60% | - |
| Caesarean | 236 | 57.10% | - |
| Assisted Vaginal Delivery | 26 | 6.30% | - |
| No of Gestation | |||
| Singleton | 392 | 94.70% | - |
| Multiple | 21 | 5.30% | - |
| Cord TSH (Mean ± SD) | 55.70 ± 18.53 | - | 37.66-100 |
| T4 (Mean ± SD) | 23.51 ± 9.79 | - | 3.76-85.70 |
Table 1. Descriptive distribution of all screened positive cases
| Year | No. of Neonates Screened | Total Positives | No. of True Positives (TP) | No of False Positives (FP) | No false Negatives (FN) | Population Incidence |
|---|---|---|---|---|---|---|
| 2012 | 4013 | 87 | 3 | 84 | 0 | 1:1337 |
| 2013 | 4034 | 95 | 4 | 87 | 0 | 1:1008 |
| 2014 | 4245 | 95 | 4 | 91 | 0 | 1:1061 |
| 2015 | 4256 | 70 | 1 | 69 | 0 | 1:4256 |
| 2016 | 4307 | 70 | 2 | 68 | 0 | 1:2153 |
| Total | 20,855 | 413 | 14 | 399 | 0 | 1:1489 |
Table 2. Population incidence of congenital hypothyroidism in Bahrain from 2012 to 2016
Table 2 shows the number of neonates screened annually and number of true positive and false positive cases. No false negative cases were observed in this study and the overall incidence of congenital hypothyroidism during the study period was 1:1489 (Figure 2).
Table 3 demonstrates CBTSH and cord blood free T4 results. Thyroid ultrasound, scintigraphy reports of all 14 confirmed cases of primary hypothyroidism.
| Cases | Nationality | Gender | Cord blood TSH | Cord FreeT4 | Ultrasound | TC99 |
|---|---|---|---|---|---|---|
| 1 | Non-Bahraini | Female | 100 | 10.04 | Normal | N/A |
| 2 | Non-Bahraini | Female | 100 | 7.23 | Not visualizes | No uptake |
| 3 | Bahraini | Male | 100 | 16.3 | Enlarged thyroid | Increased uptake |
| 4 | Bahraini | Female | 100 | 8.09 | Not visualized | No uptake |
| 5 | Non-Bahraini | Male | 100 | 6,83 | Normal size | Increase uptake |
| 6 | Non-Bahraini | Male | 100 | 8.34 | N/A | Increased uptake |
| 7 | Non-Bahraini | Male | 100 | 14.6 | N/A | No uptake |
| 8 | Bahraini | Male | 100 | 9.33 | Enlarge thyroid | Increased uptake |
| 9 | Bahraini | Male | 100 | 6.04 | N/A | N/A |
| 10 | Non-Bahraini | Female | 100 | 4.28 | N/A | N/A |
| 11 | Bahraini | Male | 94.21 | 9.96 |
Not visualized | No uptake |
| 12 | Bahraini | Male | 100 | 6.18 | Enlarged thyroid | Increased uptake |
| 13 | Non Bahraini | Female | 100 | 6.73 | N/A | N/A |
| 14 | Bahraini | Male | 100 | 3.76 | Not visualized | No uptake |
Table 3. Clinical data for 14 infants with thyroid abnormalities
Table 4 demonstrates the CBTSH distribution. There were 20,442 (98.02%) negative tests (CBTSH <37.5 μIU/mL) and 413 positive tests (CBTSH >37.5 μIU/ml) accounting for 1.98%, which was the recall rate.
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | -LR |
|---|---|---|---|---|---|---|
| = 37.66 | 100.00 | 76.8-100.0 | 0.00 | 0.0-0.9 | 1.00 | |
| >93.96 | 100.00 | 76.8-100.0 | 94.99 | 92.4-96.9 | 19.95 | 0.00 |
| >99.1 | 92.86 | 66.1-99.8 | 95.74 | 93.3-97.5 | 21.79 | 0.075 |
| >100 | 0.00 | 0.0-23.2 | 100.00 | 99.1-100.0 | 1.00 |
Table 4. TSH distribution
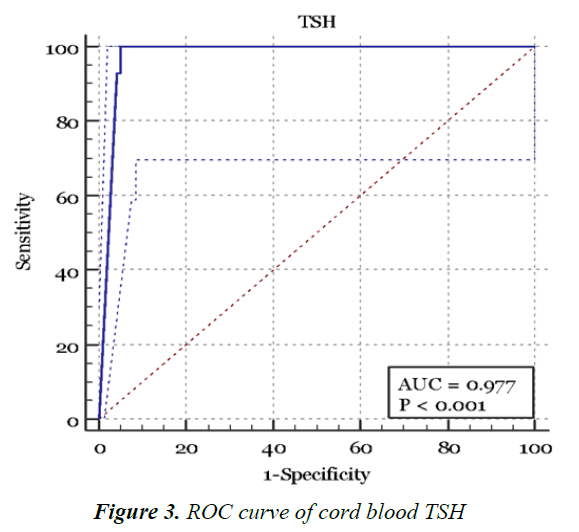
Figure 3 and Table 5 shows the ROC curve created from plotting the true positive results (sensitivity) against the false positive results (1-Specificity) [9,10]. CBTSH of 93 mU/l as the appropriate detection cutoff for the diagnosing CH with a sensitivity of 100% (95% CI, 76.8%-100%) and a specificity of 95% (95% CI, 92.4%-96.9%).
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | -LR |
|---|---|---|---|---|---|---|
| = 37.66 | 100.00 | 76.8-100.0 | 0.00 | 0.0-0.9 | 1.00 | |
| >93.96 | 100.00 | 76.8-100.0 | 94.99 | 92.4-96.9 | 19.95 | 0.00 |
| >99.1 | 92.86 | 66.1-99.8 | 95.74 | 93.3-97.5 | 21.79 | 0.075 |
| >100 | 0.00 | 0.0-23.2 | 100.00 | 99.1-100.0 | 1.00 |
Table 5. Criterion values and coordinates of the ROC curve
Discussion
The use of CBTSH as a screening tool for CH has been validated in numerous studies [11]. CBTSH values have been evaluated and compared with filter paper samples obtained via heel prick [12]. Hardy et al. [13] found CBTSH to be more sensitive than cord blood free T4 screening.
There are wide variations in screening strategies worldwide with regard to timing and methods of screening [14,15]. Primary TSH with backup T4 measurements is widely used in Europe, Japan, Canada, Mexico and the United States. Some programs in North America, Israel and the Netherlands also use primary T4 measurements and both methods use heel prick samples at 2 to 5 days after birth [5,16,17].
The use of CBTSH as a screening tool in developing Asian countries provides an attractive alternative because of its simplicity and accessibility considering the obstetric practice in which the majority of mothers are discharged within 24 to 48 h after delivery [14,18,19].
By using a cutoff value of 37.5 μIU/ml in our hospital, we identified 413 infants at risk out of a total population of 20,855 infants. Fourteen infants in the population identified at risk were confirmed after repeat TSH and free T4 as being hypothyroid. An overall incidence of 1:1489, which was higher than the previously reported incidence of 1:2967 [20] and estimated worldwide incidence of 1:3000 [21], was similar to Najran province in Saudi Arabia (1:1400), Iran (1:1439) and 1:1500 in Ontario, Canada [22-24].
Recall rates in various programs around the world range from 0.01% to 13.3% [6]. Our study had a recall rate of 1.98% compared to 0.05%–0.3% found in other primary TSH screening programs [16,25].
The lowest TSH value detected in confirmed CH cases was 94 μIU/ml, which is consistent with values from a Swedish study (100 μIU/ml) and from Najarn province in Saudi Arabia (117 μIU/ml) [26,27].
The ROC curve identified a new cutoff CBTSH value as 93 μIU/ml. When used, it would improve the efficiency of the test and lead to less false positive cases, and hence lower recall rates, which in return will significantly improve the cost-effectiveness of this screening tool and minimize concerned parents’ anxiety for whom their infants are identified as at risk [6,8].
Limitations
Study limitations included the retrospective design and use of primary TSH and backup free T4 measurements, which may miss cases of central hypothyroidism and cases with delayed TSH elevation seen in low birth weight and very low birth weight infants (LBW and VLBW, respectively) and infants with thyroid-binding globulin (TBG) deficiency. An approach using simultaneous TSH and free T4 measurements seems to be the preferred ideal method to diagnose CH [16,28].
Conclusion
To date, there is no universal TSH cutoff point to screen for CH. Each country should collect enough data to determine a credible and feasible screening method and cutoff values aiming to achieve low false positive results and recall rate. CBTSH screening per se will detect neonates with primary CH, yet cases with central hypothyroidism and those with delayed TSH elevation can be missed. Our study supports setting higher CBTSH cut-off values based on our population findings and recommends simultaneous cord free T4 measurements as the ideal screening approach.
References
- Grosse SD, Van Vliet G. Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch Dis Childhood 2011.
- https://www.uptodate.com/contents/clinical-features-and-detection-of-congenital-hypothyroidism
- Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis 2010; 5: 17.
- Dussault JH. The anecdotal history of screening for congenital hypothyroidism. J Clin Endocrinol Metab 1999; 84: 4332-4334.
- Ford G, LaFranchi SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Pract Res Clin Endocrinol Metab 2014; 28: 175-187.
- Büyükgebiz A. Newborn screening for congenital hypothyroidism. J Clin Res Pediatr Endocrinol 2013; 5: 8.
- Mehran L, Khalili D, Yarahmadi S, et al. Worldwide recall rate in newborn screening programs for congenital hypothyroidism. Int J Endocrinol Metab 2017; 15.
- Schmidt JL, Castellanos-Brown K, Childress S, et al. The impact of false-positive newborn screening results on families: A qualitative study. Genet Med 2012; 14: 76.
- Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med 2013; 4: 627.
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39: 561-577.
- Ilamaran V, Rathisharmila R, Uvaraj P, et al. Neonatal screening for congenital hypothyroidism using cord blood thyroid stimulating hormone. Curr Pediatr Res 2014; 18.
- Walfish P. Evaluation of three thyroid-function screening tests for detecting neonatal hypothyroidism. Lancet 1976; 307: 1208-1211.
- Hardy JD, Zayed R, Doss I, et al. Cord blood thyroxine and thyroid stimulating hormone screening for congenital hypothyroidism: How useful are they? J Pediatr Endocrinol Metab 2008; 21: 245-250.
- Wong SL, Jalaludin MY, Zaini AA, et al. Congenital hypothyroidism: An audit and study of different cord blood screening TSH values in a tertiary medical centre in Malaysia. Adv Endocrinol 2015.
- Léger J, Olivieri A, Donaldson M, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis and management of congenital hypothyroidism. Hormone Res Paediatr 2014; 81: 80-103.
- American Academy of Pediatrics. American Academy of Pediatrics. AAP Section on Endocrinology and Committee on Genetics and American Thyroid Association Committee on Public Health: Newborn screening for congenital hypothyroidism: recommended guidelines. Pediatrics 1993; 91: 1203-1209.
- Madison LD, LaFranchi S. Screening for congenital hypothyroidism: Current controversies. Curr Opin Endocrinol Diabetes Obes 2005; 12: 36-41.
- Manglik AK, Chatterjee N, Ghosh G. Umbilical cord blood TSH levels in term neonates: a screening tool for congenital hypothyroidism. Indian Pediatr 2005; 42: 1029-1032.
- Al-Jurayyan NA, Al-Nuaim AA, El-Desouki MI, et al. Neonatal screening for congenital hypothyroidism in Saudi Arabia: Results of screening the first 1 million newborns. Screening 1996; 4: 213-220.
- Golbahar J, Al-Khayyat H, Hassan B, et al. Neonatal screening for congenital hypothyroidism: A retrospective hospital based study from Bahrain. J Pediatr Endocrinol Metab 2010; 23: 39-44.
- LaFranchi SH. Worldwide coverage of newborn screening for congenital hypothyroidism - A public health challenge. Alm 2014; 2: S225-233.
- Al-Jurayyan NA, Shaheen FI, Al-Nuaim AA, et al. Congenital hypothyroidism: Increased incidence in Najran province, Saudi Arabia. J Trop Pediatr 1996; 42: 348-351.
- Ordookhani A, Mirmiran P, Moharamzadeh M, et al. A high prevalence of consanguineous and severe congenital hypothyroidism in an Iranian population. J Pediatr Endocrinol Metabol 2004; 17: 1201-1210.
- Saleh DS, Lawrence S, Geraghty MT, et al. Prediction of congenital hypothyroidism based on initial screening thyroid-stimulating-hormone. BMC Pediatr 2016; 16: 24.
- Walfish PG, Ginsberg J, Rosenberg RA, et al. Results of a regional cord blood screening programme for detecting neonatal hypothyroidism. Arch Dis Childhood 1979; 54: 171-177.
- Alm J, Hagenfeldt L, Larsson A, et al. Incidence of congenital hypothyroidism: Retrospective study of neonatal laboratory screening versus clinical symptoms as indicators leading to diagnosis. Br Med J 1984; 289: 1171-1175.
- Ogunkeye OO, Roluga AI, Khan FA. Resetting the detection level of cord blood thyroid stimulating hormone (TSH) for the diagnosis of congenital hypothyroidism. J Trop Pediatr 2007; 54: 74-77.
- Rose SR, Brown RS; American Academy of Pediatrics, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006; 117: 2290-2303.