Research Article - Journal of Clinical Ophthalmology (2020) Volume 4, Issue 3
Real-world evaluation of postoperative in-office Dexamethasone intracanalicular insert administration for control of postoperative inflammation following cataract surgery
P Dee G Stephenson MD, FACS*
Stephenson Eye Associates, 200 Palermo Place, Venice, FL, USA- Corresponding Author:
- Mohammadkarim Johari
Department of Ophthalmology Shiraz University of Medical Sciences Shiraz Iran
E-mail: mkjoharii@gmail.com
Accepted date: June 22, 2020
Citation: Stephenson PDG. Real-world evaluation of postoperative in-office Dexamethasone intracanalicular insert administration for control of postoperative inflammation following cataract surgery. J Clin Ophthalmol 2020;4(3):273-278.
Abstract
Background: The purpose of this study is to describe outcomes with in-office postoperative use of the dexamethasone intracanalicular insert for control of postoperative inflammation following cataract surgery when administered in the office on the first postoperative day.
Methods: Data from consecutive patients undergoing uncomplicated femtosecond-assisted phacoemulsification were retrospectively drawn from electronic health records. All eyes received in-office placement of a single dexamethasone insert into the inferior punctum of the operative eye on the first postoperative day. Pain was graded as none/mild/moderate/severe and anterior chamber cells and flare were graded using the Standardization of Uveitis Nomenclature Working Group’s standardized schema preoperatively and 1 day, 1-2 weeks, and 6-8 weeks postoperatively. Intraocular pressure (IOP) data, as well as adverse events, were also recorded at all time points.
Results: Overall, data from 17 eyes of 12 patients were included in this analysis. All eyes had modest amounts of cells and flare in the anterior chamber on the first postoperative day before dexamethasone insert placement. By the week 1-2 visit (mean 12 days postoperatively) and again at the week 6-8 visit (mean 51 days postoperatively), no eyes had any cells or flare (0%). The insert was visualized in all eyes at week 1-2 and had dissolved completely in 16/17 eyes (94.1%) by week 6-8. No clinically significant IOP elevations occurred in any eyes and mean IOP was significantly reduced throughout follow-up.
Conclusions: Real-world experience with the dexamethasone intracanalicular insert placed in-office on the first postoperative day following cataract surgery is consistent with findings from previous clinical trials and demonstrated that the therapy safely and effectively controlled postoperative inflammation in combination with topical NSAID therapy. This product offers surgeons the opportunity to optimize adherence with anti-inflammatory therapy, while reducing patients’ postoperative topical therapy burden, after cataract surgery.
Keywords
Cataract surgery, Dexamethasone, Dextenza, Pain, Postoperative inflammation, Sustained release.
Abbreviations:
BCVA: Best-Corrected Visual Acuity; CME: Cystoid Macular Edema; FBS: Foreign Body Sensation; IOP:Intraocular Pressure; logMAR: Logarithm of the Minimum Angle of Resolution; mmHg: Millimeters of Mercury; MUST trial: Multicenter Uveitis Steroid Treatment trial; NSAID: Non-steroidal Anti-Inflammatory Drug; SE: Standard Error; US: United States; WIRB: Western Institutional Review Board.
Background
Ocular inflammation – characterized by the presence of cells and/or flare in the anterior chamber – is nearly ubiquitous following cataract surgery, occurring in 95% or more of eyes with or without perioperative anti-inflammatory prophylaxis as soon as the first postoperative day [1]. Consequences of iatrogenic inflammation include pain [2-4], increased intraocular pressure, the formation of anterior and posterior iris synechiae [5-7], and postoperative cystoid macular edema (CME) [8,9], all of which contribute to patient discomfort, delayed recovery, and reduced visual outcomes [9,10].
Perioperative anti-inflammatory therapy, consisting of topical corticosteroids, topical non-steroidal anti-inflammatory drugs (NSAIDs), or both, is routinely prescribed for patients undergoing cataract surgery [11]. Concurrent topical antibiotics are prescribed for infection prophylaxis in more than half of cataract cases in the US [11]. Adding to the complexity of a 3- drug dosing regimen is the variable-dose taper of corticosteroids over time. Regimen complexity has been associated with poor adherence to topical ophthalmic therapy [12-17]. An adherence study of patients undergoing cataract surgery who were prescribed topical prednisolone and gentamicin five times daily for 14 days revealed that most patients were sub-optimally adherent; half of patients were less than 50% adherent with all doses and only 5% administered at least 75% of prescribed doses [18].
Recently, the United States Food and Drug Administration approved 2 sustained-release formulations of dexamethasone for control of postoperative inflammation and pain following cataract surgery. One is a suspension intended for intraocular administration at the time of surgery (Dexycu, dexamethasone 517 μg, EyePoint Pharmaceuticals, Watertown, MA), while the other is an intracanalicular insert (Dextenza, dexamethasone 0.4 mg, Ocular Therapeutix, Bedford, MA). These therapeutic options are surgeon-administered and obviate the need for patient adherence.
In their phase 3 registry trials, the dexamethasone products described above were administered in the operating room at the completion of surgery [19-21]. In this report, we describe the effects of the dexamethasone 0.4 mg intracanalicular insert (Dextenza) when placed in the office setting on the first postoperative day on postsurgical inflammation and intraocular pressure (IOP) in a series of patients undergoing routine elective cataract surgery.
Methods
This was a retrospective analysis of existing health records. The protocol for data collection was reviewed and approved by the Western Institutional Review Board (WIRB) ethics committee on 12 December 2019, and a waiver of consent was granted.
Subjects were consecutive adults age 18 years or older with visually significant cataract undergoing elective femtosecond laser-assisted cataract extraction by a single surgeon between July and September 2019 with in-office placement of a dexamethasone 0.4 mg intracanalicular insert on the first postoperative day. Subjects were excluded if any intraoperative complications occurred or if the dexamethasone insert was not administered on the first postoperative day.
The surgical procedure included standard use of the femtosecond laser for creation of the corneal incision, anterior capsulorhexis, and lens fragmentation, followed by standard phacoemulsification for lens nucleus removal, irrigation/ aspiration of residual cortex, and intraocular lens implantation. All eyes received a single dexamethasone insert, a polyethelene glycol hydrogel rod impregnated with 0.4 mg of preservative-free dexamethasone, conjugated with fluorescein for visualization, that is inserted through the punctum into the canaliculus where is self-anchors by swelling upon hydration and dissolves over time with sustained and tapered delivery of dexamethasone for up to 30 days [19]. It can be inserted preoperatively, intraoperatively, or postoperatively at surgeons’ discretion. In the current study, the dexamethasone insert was placed in the inferior punctum of the operated eye, as per the product’s instructions for use [22],at the completion of the postoperative day 1 visit. The insert was positioned just below the level of the punctal opening, and 1-2 drops of artificial tears were instilled to hydrate the insert. Proper positioning of the insert was confirmed by visual inspection at the slit-lamp immediately after placing and at each postoperative visit thereafter using blue light illumination. All subjects received a standardized periocular medical regimen consisting of hypochlorous acid spray twice daily to the eyelids beginning 2 weeks before and continuing 2 weeks after surgery, as well as bromfenac and besifloxacin each dosed twice daily beginning 3 days before surgery and continuing until postoperative day 42.
Data collected from the electronic health records of consecutive eligible subjects included demographic information, ocular history, and intraocular pressure (IOP) preoperatively, surgical complications intraoperatively, and visualization of the insert, IOP, presence of anterior chamber cell or flare, the need for additional therapy for pain or inflammation management beyond the routine postoperative regimen, and adverse events on postoperative day 1, week 1-2, and week 6-8, as well as the number and nature of issues raised by patients via phone calls between scheduled visits. Insert visualization was scored dichotomously as present or absent based on inspection of the punctum/canaliculus at the slitlamp. Intraocular pressure was measured using Goldmann tonometry. Anterior chamber cell and flare were quantified using the Standardization of Uveitis Nomenclature Working Group’s standardized grading schema (cell graded as 0, 0.5+, or 1-4+; flare graded as 0 or 1-4+) [23].
The primary efficacy outcome was resolution of anterior chamber cells and flare. Secondary outcomes included adverse events, IOP elevations>10 mmHg above baseline IOP at any of the 3 postoperative visits, the proportion of eyes requiring additional anti-inflammatory therapy beyond the routine regimen, and the incidence and nature of between-visit patientreported issues. Analysis of data was limited to descriptive statistics. As no hypotheses were being tested, no formal power or sample size calculations were performed a priori.
Results
Data were collected from cataract surgery in 17 eyes of 12 subjects. Demographic and baseline data are given in Table 1. Subjects’ average age was 71.2 (standard error 1.7) years, all were Caucasian, and males slightly outnumbered females (7 males, 5 females). Ten right eyes and 7 left eyes were operated upon.
| Parameter | Value |
|---|---|
| Subject-level (n=12) | |
| Age (yr), mean (SE) | 71.2 (1.7) |
| Ethnicity (% Caucasian) | 100 |
| Gender, n (%) | |
| Male | 7 (58.3) |
| Female | 5 (41.7) |
| Eye-level (n=17) | |
| Operative eye, n (%) | |
| Right eye | 10 (58.8) |
| Left eye | 7 (41.2) |
| Preoperative logMAR BCVA, mean (SE) | 0.54 (0.09) |
| Preoperative IOP (mmHg), mean (SE) | 18.9 (0.6) |
| Proportion of eyes with cell present at baseline (%) | 0 |
| Proportion of eyes with flare present at baseline (%) | 0 |
*BCVA: Best-Corrected Visual Acuity; IOP: Intraocular Pressure; logMAR: logarithm of the Minimum Angle of Resolution; SE: Standard Error.
Table 1 : Demographic and baseline data for the study sample (n=17 eyes, n=12 subjects).
All eyes underwent uncomplicated femtosecond-assisted phacoemulsification. The dexamethasone insert was placed inoffice at the completion of the postoperative day 1 visit without complication in all 17 eyes. The insert was visualized at the week 1-2 visit in all eyes and was completely resorbed in all but 1 eye (16/17; 94.1%) by the week 6-8 visit (Table 2).
| Day 1 | Week 1-2 | Week 6-8 | |
|---|---|---|---|
| Days since surgery, mean (SE) | 1 (0) | 12 (1) | 51 (3) |
| Anterior chamber cell, n (%) | |||
| Any | 17 (100) | 0 (0) | 0 (0) |
| 0.5+ | 16 (94.1) | 0 (0) | 0 (0) |
| 1+ | 1 (5.9) | 0 (0) | 0 (0) |
| Anterior chamber flare, n (%) | |||
| Any | 17 (100) | 0 (0) | 0 (0) |
| 1+ | 16 (94.1) | 0 (0) | 0 (0) |
| 2+ | 1 (5.9) | 0 (0) | 0 (0) |
*SE: Standard Error
Table 2 : Anterior chamber cell and flare data at each postoperative time point (n=17 eyes).
Cell and flare data are given in Table 2. No eyes had any cell or flare preoperatively. On postoperative day 1, 94.1% of eyes (16/17) had 0.5+ cell (1-5 cells per high-power field) and 5.9% (1/17) had 1+ cell (6-15 cells per high-power field) in the anterior chamber. Similarly, 94.1% of eyes (16/17) had 1+ flare (faint) and 5.9% (1/17) had 2+ flare (moderate with iris/lens details clear). The same eye had 1+ cell and 2+ flare. By week 1-2, and also at week 6-8, no eyes had any discernable anterior chamber cells (0/17) or flare (0/17) (Table 2).
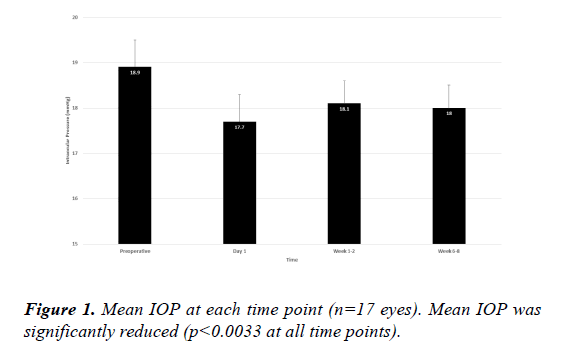
No eyes experienced an IOP elevation of>10 mmHg from baseline at any postoperative time point. Mean IOP at baseline (Figure 1) was 18.9 (0.6) mmHg and was significantly lower at postoperative day 1 (17.7 [0.6] mmHg; p=0.0032), week 1-2 (18.1 [0.5] mmHg; p=0.0032), and week 6-8 (18.0 [0.5] mmHg; p=0.0025). The maximum rise in IOP from preoperatively to peak postoperative IOP was 2.0 mmHg in a single eye.
Best-corrected visual acuity improved in all eyes from preoperatively to the final postoperative visit, from a mean of 0.54 (0.09) logMAR to a mean of 0.08 (0.06) logMAR (p<0.00101). All but 2 eyes achieved final BCVA of 20/20; the remaining 2 eyes of the same patient with a history of bilateral traumatic optic neuropathy each had preoperative BVCA 20/400 [1.3 logMAR]) and each achieved final BCVA of 20/100.
No adverse events related to surgery or the dexamethasone insert were noted at any visits. Three patients reported a total of five adverse events via phone between visits (Table 3). Each of these 3 patients reported foreign body sensation, one each on postoperative day 1 (after returning home from the postoperative day 1 visit at which the insert was placed), day 24, and day 27. In all 3 cases, artificial tears were recommended. The patient with foreign body sensation on the day of insertion called back the next day with persistent symptoms, was seen in the office, and found to have minor extrusion of the insert through the punctum; this was repositioned without complication at the slit-lamp with resolution of symptoms. The remaining two cases of foreign body sensation resolved with use of artificial tears. One of these two patients called back two weeks later (postoperative day 42) to report dryness in the operative eye that resolved with increased use of artificial tears. No patients reported any pain in the operative eye at any postoperative visit.
| Subject | Postoperative Day of Event | Nature of Event | Intervention | Resolution |
|---|---|---|---|---|
| 11 | 24 | FBS | Artificial tears | Resolved |
| 42 | Dryness | Artificial tears | Resolved | |
| 14 | 28 | FBS | Artificial tears | Resolved |
| 15 | 1 | FBS | Artificial tears | Did not resolve |
| 2 | FBS | Patient evaluated; insert repositioned | Resolved |
*FBS: Foreign Body Sensation
Table 3 : Adverse events reported by phone.
Discussion
In this series of 17 eyes undergoing routine cataract surgery, inoffice intracanalicular administration of the dexamethasone 0.4 mg insert on postoperative day 1, in combination with topical NSAID and antibiotic use, resulted in resolution of anterior chamber cells and flare by postoperative weeks 1-2 with excellent visual rehabilitation and no adverse effects on intraocular pressure.
These results are consistent with the findings of three phase 3 clinical studies evaluating the effect of the Dextenza intracanalicular dexamethasone insert on anterior chamber cells following cataract surgery. In these studies, patients undergoing elective cataract surgery were administered either the dexamethasone insert or an inert vehicle immediately following surgery in the operating room; eyes receiving the active dexamethasone insert were consistently more likely to have absence of cells at day 14 compared to vehicle (33.1%-52.3% of dexamethasone eyes and 14.5%-31.3% of vehicle eyes; differences significant in 2 of 3 studies) [19,20]. The higher rate of cell clearance in the current study (100% by Week 1-2 [average 12 days postoperatively]) may be related to the addition of postoperative topical NSAID therapy in our series; NSAIDs were not used routinely in the phase 3 studies.
The insert was well tolerated by most patients. Overall, 3 patients reported 5 episodes of foreign body sensation or dryness all but one of which resolved with conservative measures; the relationship of these events to the insert cannot be established as the nature of the events are common following cataract surgery without insert placement. An unscheduled patient visit was only necessary in 1 case (1/17, 5.9%); the issue was symptomatic migration of the insert shortly after administration and was easily remedied by repositioning the insert. This rate is consistent with prior reports of problem-based unscheduled visit rates following cataract surgery in the range of 3%-4% [24]. No clinically significant IOP elevations were seen in this series. In phase 3 studies, the incidence of IOP elevations>10 mmHg ranged from 4.4%-6.8% in dexamethasone eyes and 3.6%-5.0% in control eyes; almost all IOP elevations occurred within the first 2 days postoperatively and were attributed to the surgical procedure rather than the dexamethasone insert [19,20]. In this study, IOP was significantly reduced at every postoperative point, consistent with known effects of phacoemulsification on IOP [25,26].
The dexamethasone insert is also approved for the control of postoperative pain. No patients in this study reported any pain at any postoperative visit, including the first postoperative day, thus precluding assessment of pain control in this sample.
The development of surgeon-administered sustained-delivery corticosteroid formulations addresses important unmet needs for perioperative cataract surgery therapy. Perhaps most importantly, the need for patient adherence to self-dosing of a tapering steroid eye drop regimen is eliminated. This is impactful for several reasons. Not only are many doses of perioperative anti-inflammatory therapy and antimicrobial therapy not administered by patients as prescribed [18], the doses that are administered are often done so incorrectly. Up to 92% of postoperative anti-inflammatory eye drop instillations are performed incorrectly (missing the eye, delivering the wrong dose, contact between the bottle and the ocular surface) [27].Direct ocular trauma related to contact between the bottle tip and the eye can result in conjunctival and corneal abrasions in nearly 70% of elderly patients [28] (the population typically undergoing cataract surgery) which can predispose to wound leakage and postoperative infection [29].
Dropless cataract surgery is an emerging concept in the modern era as a means of improving outcomes and the patient experience overall by reducing or eliminating the need for patient administration of anti-inflammatory and antimicrobial therapy in the perioperative cataract surgery period [30,31]. Eliminating multiple drops per day from multiple bottles for multiple weeks has several potential benefits. In addition to the adherence issues discussed above, a drop-free postoperative course spares the ocular surface exposure to the preservatives in eye drop formulation, which can make ocular surface disease worse [32-34]. Of note, both of the dexamethasone sustained release formulations are preservative-free [22,35]. Combined with emerging evidence supporting the greater efficacy of intraoperative intracameral antibiotics over postoperative topical antibiotics in preventing postoperative endophthalmitis [36-38],the option of surgeon-administered anti-inflammatory and antimicrobial therapy moves the cataract surgery patient experience one step closer to a dropfree postoperative course.
This is the first real-world study of which we are aware describing clinical outcomes of the dexamethasone intracanalicular insert placed in-office during the postoperative period around cataract surgery. As such, it complements the results of clinical trials summarized above and provides additional insight into the use of this product in routine clinical practice outside the narrow constraints of registry trials and administered at a different time than in these clinical trials (inoffice on the first postoperative day versus end-of-surgery on the surgical day). The small sample size is a limitation of the study; the possibility of selection bias was mitigated by enrolling consecutive subjects within a narrow enrollment window. The lack of a control group is also a limitation. As this precluded masking of the investigator when grading anterior chamber cell and flare, effort was made to minimize subjectivity by utilizing a well-validated anterior chamber cell and flare grading schema developed by the Standardization of Uveitis Nomenclature Working Group [23] and used in the landmark NIH-funded Multicenter Uveitis Steroid Treatment (MUST) trial [39].
Conclusion
In summary, real-world experience with the dexamethasone intracanalicular insert placed in-office on the first postoperative day following cataract surgery demonstrated that the therapy safely and effectively controlled postoperative inflammation in combination with topical NSAID therapy. This product offers surgeons the opportunity to optimize adherence with antiinflammatory therapy, while reducing patients’ postoperative topical therapy burden, after cataract surgery.
Declarations
Ethics approval and consent to participate
This study ’ s protocol was reviewed and approved by the Western IRB and a waiver of consent was issued for retrospective data collection. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request..
Competing interests
The author declares that they have no competing interests.
Funding
Sponsorship for this study was funded by Ocular Therapeutix, Inc.
Author’s contributions
PDS was the sole author and analyzed and interpreted the data and oversaw the writing of the manuscript.
Acknowledgements
Editorial assistance in the preparation of this article was provided by Tony Realini, MD, MPH of Hypotony Holdings LLC. Support for this assistance was provided by Ocular Therapeutix.
References
- Walters TR, Goldberg DF, Peace JH, et al. Bromfenac Ophthalmic Solution 0.07% Dosed Once Daily for Cataract Surgery: Results of 2 Randomized Controlled Trials. Ophthalmology. 2014;121:25-33.
- Porela-Tiihonen S, Kokki H, Kaarniranta K, et al. Recovery after Cataract Surgery. ActaOphthalmol. 2016;94:1-34.
- Porela-Tiihonen S, Kaarniranta K, Kokki M, et al. A Prospective Study on Postoperative Pain after Cataract Surgery. ClinOphthalmol. 2013;7:1429-35.
- Porela-Tiihonen S, Kaarniranta K, Kokki H. Postoperative Pain after Cataract Surgery. J Cataract Refract Surg. 2013;39:789-98.
- Dick HB, Schwenn O, Krummenauer F, et al. Inflammation after Sclerocorneal Versus Clear Corneal Tunnel Phacoemulsification. Ophthalmology. 2000;107:241-7.
- Mohammadpour M, Jafarinasab MR, Javadi MA. Outcomes of Acute Postoperative Inflammation after Cataract Surgery. Eur J Ophthalmol. 2007;17:20-8.
- Ianchulev T, Litoff D, Ellinger D, et al. Office-Based Cataract Surgery: Population Health Outcomes Study of More Than 21 000 Cases in the United States. Ophthalmology. 2016;123:723-8.
- Porela-Tiihonen S. Recovery after Cataract Surgery. ActaOphthalmol. 2016;94:523-4.
- Dua HS, Attre R. Treatment of Post-Operative Inflammation Following Cataract Surgery – a Review. Eur Ophthalmic Rev. 2012;6:98-103.
- McColgin AZ, Heier JS. Control of Intraocular Inflammation Associated with Cataract Surgery. CurrOpinOphthalmol. 2000;11:3-6.
- Zafar S, Wang P, Schein OD, et al. Prescribing Patterns and Costs Associated with Postoperative Eye Drop Use in Medicare Beneficiaries Undergoing Cataract Surgery. Ophthalmology. 2020;127:573-81.
- Tsai JC. A Comprehensive Perspective on Patient Adherence to Topical Glaucoma Therapy. Ophthalmology. 2009;116:S30-6.
- Tsai JC, McClure CA, Ramos SE, et al. Compliance Barriers in Glaucoma: A Systematic Classification. J Glaucoma. 2003;12:393-8.
- Sleath B, Carpenter DM, Blalock SJ, et al. Applying the Resources and Supports in Self-Management Framework to Examine Ophthalmologist-Patient Communication and Glaucoma Medication Adherence. Health Educ Res. 2015;30:693-705.
- Robin AL, Covert D. Does Adjunctive Glaucoma Therapy Affect Adherence to the Initial Primary Therapy? Ophthalmology. 2005;112:863-8.
- McClelland JF, Bodle L, Little JA. Investigation of Medication Adherence and Reasons for Poor Adherence in Patients on Long-Term Glaucoma Treatment Regimes. Patient Prefer Adherence. 2019;13:431-9.
- Newman-Casey PA, Robin AL, Blachley T, et al. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology. 2015;122:1308-16.
- Hermann MM, Ustundag C, Diestelhorst M. Electronic Compliance Monitoring of Topical Treatment after Ophthalmic Surgery. IntOphthalmol. 2010;30:385-90.
- Walters T, Bafna S, Vold S, et al. Efficacy and Safety of Sustained Release Dexamethasone for the Treatment of Ocular Pain and Inflammation after Cataract Surgery: Results from Two Phase 3 Studies. J ClinExpOphthalmol. 2016;7:1-11.
- Tyson SL, Bafna S, Gira JP, et al. Multicenter Randomized Phase 3 Study of a Sustained-Release Intracanalicular Dexamethasone Insert for Treatment of Ocular Inflammation and Pain after Cataract Surgery. J Cataract Refract Surg. 2019;45:204-12.
- Donnenfeld E, Holland E. Dexamethasone Intracameral Drug-Delivery Suspension for Inflammation Associated with Cataract Surgery: A Randomized, Placebo-Controlled, Phase Iii Trial. Ophthalmology 2018;125:799-806.
- Ocular Therapeutix, Inc. Dextenza. Highlights of Prescribing Information 2020.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature for Reporting Clinical Data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-16.
- Eloranta H, Falck A. Is an Ophthalmic Check-up Needed after Uneventful Cataract Surgery? A Large Retrospective Comparative Cohort Study of Finnish Patients. ActaOphthalmol. 2017;95:665-70.
- Sengupta S, Venkatesh R, Krishnamurthy P, et al. Intraocular Pressure Reduction after Phacoemulsification Versus Manual Small-Incision Cataract Surgery: A Randomized Controlled Trial. Ophthalmology. 2016;123:1695-703.
- Baek SU, Kwon S, Park IW, et al. Effect of Phacoemulsification on Intraocular Pressure in Healthy Subjects and Glaucoma Patients. J Korean Med Sci. 2019;34:e47.
- An JA, Kasner O, Samek DA, et al. Evaluation of Eyedrop Administration by Inexperienced Patients after Cataract Surgery. J Cataract Refract Surg. 2014;40:1857-61.
- Dietlein TS, Jordan JF, Luke C, et al. Self-Application of Single-Use Eyedrop Containers in an Elderly Population: Comparisons with Standard Eyedrop Bottle and with Younger Patients. ActaOphthalmol. 2008;86:856-9.
- Taban M, Sarayba MA, Ignacio TS, et al. Ingress of India Ink into the Anterior Chamber through Sutureless Clear Corneal Cataract Wounds. Arch Ophthalmol. 2005;123:643-8.
- Lindstrom RL, Galloway MS, Grzybowski A, et al. Dropless Cataract Surgery: An Overview. Curr Pharm Des. 2017;23:558-64.
- Shorstein NH, Myers WG. Drop-Free Approaches for Cataract Surgery. CurrOpinOphthalmol. 2020;31:67-73.
- Rosin LM, Bell NP. Preservative Toxicity in Glaucoma Medication: Clinical Evaluation of Benzalkonium Chloride-Free 0.5% Timolol Eye Drops. ClinOphthalmol. 2013;7:2131-5.
- Maca SM, Amon M, Findl O, et al. Efficacy and Tolerability of Preservative-Free and Preserved Diclofenac and Preserved Ketorolac Eyedrops after Cataract Surgery. Am J Ophthalmol. 2010;149:777-84.
- Kasetsuwan N, Satitpitakul V, Changul T, et al. Incidence and Pattern of Dry Eye after Cataract Surgery. PLoS ONE 2013;8:e78657.
- Eyepoint Pharmaceuticals. Dexycu Highlights of Prescribing Information 2020.
- Bowen RC, Zhou AX, Bondalapati S, et al. Comparative Analysis of the Safety and Efficacy of Intracameral Cefuroxime, Moxifloxacin and Vancomycin at the End of Cataract Surgery: A Meta-Analysis. Br J Ophthalmol. 2018;102:1268-76.
- Huang J, Wang X, Chen X, et al. Perioperative Antibiotics to Prevent Acute Endophthalmitis after Ophthalmic Surgery: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0166141.
- Kessel L, Flesner P, Andresen J, et al. Antibiotic Prevention of Postcataract Endophthalmitis: A Systematic Review and Meta-Analysis. ActaOphthalmol. 2015;93:303-17.
- Multicenter Uveitis Steroid Treatment Trial Research Group, Kempen JH, Altaweel MM, et al. The Multicenter Uveitis Steroid Treatment Trial: Rationale, Design, and Baseline Characteristics. Am J Ophthalmol. 2010;149:550-61 e10.