Research Article - Biomedical Research (2017) Volume 28, Issue 12
Rat model of abnormal phlegmatic syndrome with erectile dysfunction based on Uygur medicine
Adilijiang Yiming1#, Fengxia Liu1#, Yuhong Jing2, Panpan Zhang1, Qiang Fan1 and Halmurat Upur3*
1Department of Human Anatomy, Xinjiang Medical University, Urumqi, P R China
2Department of Human Anatomy and Histoembryology, Lanzhou University, Lanzhou, PR China
3Department of Uyghur Traditional Medicine, Xinjiang Medical University, Urumqi, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Halmurat Upur
Department of Uyghur Traditional Medicine
Xinjiang Medical University, PR China
Accepted on April 18, 2017
Abstract
The present study is to establish a rat model of abnormal phlegmatic syndrome with Erectile Dysfunction (ED) according to the theory of Uygur traditional medicine. In addition, the feasibility and reliability of this model is evaluated. A total of 100 mature male Sprague-Dawley rats were divided into 2 groups: normal control group (n=10) that had normal environment and diet, and model group (n=90) that was treated with spinach and coriander diet in a cold-wet environment. Biological parameters and sexual behavior of the rats were observed for 20 weeks until establishment of abnormal phlegmatic syndrome and ED model. Through erection test, the rats were randomly divided into 6 groups and disproved for 2 weeks. The levels of testosterone, luteinizing hormones, follicle stimulating hormone, estradiol and prolactin in the serum of the rats were measured using enzyme-linked immunosorbent assay. The abnormal phlegmatic syndrome and ED model rats showed inactivity, dull hair, clear urine, loose and soft stool, dark tongue, greasy tongue coating, decreased body weight and water consumption, and increased food consumption, urine and stool production. For sexual behaviors, increased mounting, erectile, insertion, and ejaculation latency and decreased mounting, erectile, insertion, and ejaculation numbers were observed in ED model rats. After 2 weeks of disproof treatment, biological parameters, sexual behavior and sexual hormones were reversed. The present study demonstrates that the model mimics the development of abnormal phlegmatic syndrome with ED and shows Biological characterization consistent with clinic patients. Moreover, the model can be reversed after treatment.
Keywords
Abnormal phlegmatic syndrome, Erectile dysfunction, Rat model, Uyghur medicine.s
Introduction
Erectile Dysfunction (ED), the inability to develop or maintain sufficient erection, is widespread globally, and in developed countries, a common and multifactorial disorder that primarily affects men older than 40 years of age [1,2]. It is predicted that the worldwide prevalence of ED will reach 322 million cases by the year 2025 [3,4]. Although Phosphodiesterase (PDE5) inhibitors, such as sildenafil, vardenafil and tadalafil, can effectively treat patients with ED, improve the symptoms and quality of sexual life, but they can also cause some adverse side effects that reduce the suitability for some patients [5,6]. Therefore, there is an urgent need to develop novel and effective strategies for the treatment of ED. However, alternative treatment options need to be explored and such efforts require a better understanding of the pathological process and molecular mechanism [7,8].
Uyghur medicine has unique and certain curative effects in the treatment of male diseases, especially ED [9]. It shares an origin with Greco-Arab medicine, and describes the health of a human body as the dynamic homeostasis of four normal Hilits known as Kan, Phlegm, Sapra, and Savda [10]. The internal and external factors can destroy the balance of the four body fluids and induce abnormal Hilit, leads to the development of a corresponding syndrome coupled with different diseases [11,12].
On the basis of Hilits theory of Uyghur medicine, ED is divided into abnormal phlegmatic ED, abnormal Savda ED, abnormal Sapra ED and abnormal Kan ED, among which abnormal phlegmatic ED is a kind of common systemic disease. Abnormal phlegmatic ED is mainly caused by phlegmatic accumulation via intaking a lot of wet and cold food, living and working in wet and cold conditions, and lacking body exercises. Then, metabolites retention-induced neurological disorders, drowsiness, white greasy tongue coating, and decreased libido-abnormal phlegmatic syndrome develop, and at last lead to pathological changes and ED [13,14]. Due to the development of scientific research on Uyghur medicine in recent years, establishment of ED animal model is needed. In the present study, we create a rat model by using the theory of Uyghur medicine, in which abnormal phlegmatic syndrome is built up in rat body and finally leads to ED. In addition, we assessed the model of abnormal phlegmatic syndrome with ED by testing biological parameters, sexual behavior and the levels of sexual hormones.
Materials and Methods
Animals
One hundred healthy, adult, male Sprague-Dawley rats (grade SPF, 2-month-old, 220 ± 17 g) and 40 female rats (same condition as male, used for mating test) were obtained from Xinjiang Medical University Laboratory Animal Research Center (Urumqi, China). All male rats had normal erectile function confirmed by mating experiments. The experimental use of animals was approved by the Xinjiang Medical University Animal Care and Use Committee (Urumqi, China).
For the frequent use of the same females for testing the mating behavior of the male rats, we removed the bilateral ovaries. Briefly, the female rats were anaesthetized with 1% pentobarbital sodium (50 mg/kg) during the operation, and then intramuscular penicillin (2000 U/kg/day) was given for 7 days to prevent inflammation. After 2 weeks of resting, the female rats were prepared for mating test by injecting estradiol benzoate (20 μg/day/rat for 2 days; #090103, Tongyong, Shanghai, China) and progesterone (500 μg/rat, 4 h before mating test; #090903, Xianju, Zhejiang, China).
Three days before ED model establishment, the rats were housed at a density of 5/cage in a room with normal environment, where the Intelligence Artificial Climate Box (RQH-350; Jinghong, Shanghai, China) was located. For normal control group (N), 10 rats were randomly chosen from 100 rats, and bred in normal condition with 500 ml water, 300 g food/day and 80 g bedding material/cage. The other 90 rats (model group) were bred with the same amount of water, food (feed contained 30% of spinach and coriander) and bedding materials, but in a cold and wet environment (Intelligence Artificial Climate Box with 6°C, 85%-95% humidity) for 12 h per day. Before further classifying the model group, relevant biological parameters including body weight, food consumption, water consumption, urine production and stool production were measured daily until abnormal phlegmatic syndrome (inactivity, dull hair, clear urine, loose and soft stool, dark tongue, greasy tongue coating, decreased body weight and water consumption, increased food consumption, urine and stool production) appeared, after approximately 20 weeks.
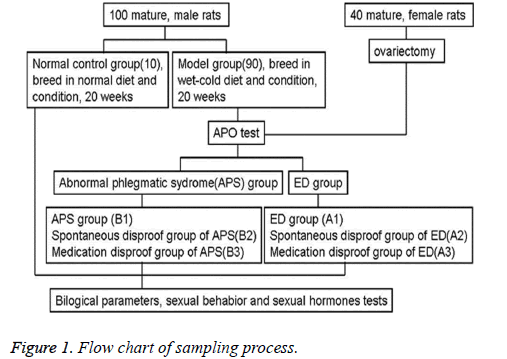
Through apomorphine (APO; Sigma-Aldrich, St. Louis, MO, USA) erectile test, the abnormal phlegmatic syndrome model rats were randomly divided into 6 different groups: abnormal phlegmatic syndrome group (B1, normal APO erectile test, contagiously lived in the same experimental condition); spontaneous disproof group of abnormal phlegmatic syndrome (B2, normal APO erectile test, moved to normal condition with normal diet); medication disproof group of abnormal phlegmatic syndrome (B3, normal APO erectile test, moved to normal condition with normal diet plus anti-ED drug); ED model group (A1, abnormal APO erectile test, lived in the same experimental condition); spontaneous disproof group of ED model (A2, abnormal APO erectile test, moved to normal condition with normal diet); medication disproof group of ED model (A3, abnormal APO erectile test, moved to normal condition with normal diet plus anti-ED drug). After two weeks of treatment, we measured the biological parameters, sexual behavior and sexual hormone levels of the groups (Figure 1).
Detection of sexual behavior
Two female rats and one male rat were kept in a new transparent cage with a cover lid for 30 min to observe the sexual behavior. Ten minutes of adaptation time was given under quiet and dim condition before starting measuring the latency of mounting, erectile, insertion, and ejaculation, and the number of mounting, erectile, insertion, and ejaculation.
Enzyme-linked immunosorbent assay (ELISA)
After anesthesia, the rats were made to bleed through their cut jugular veins which were slightly displaced (to prevent contamination of the blood by interstitial fluid) into clean, dry centrifuge tubes. The blood was left for 10 min at room temperature to clot. The tubes were then centrifuged at 33.5 Xg for 15 min within 1 h after collection. The sera were later aspirated with Pasteur pipettes into sample bottles and used within 12 h of preparation. The remaining sera were kept frozen at -80°C before being used for other biochemical assays. The levels of Testosterone (T), Luteinizing Hormones (LH), Follicle Stimulating Hormone (FSH), Estradiol (E2) and Prolactin (PRL) in the serum of the rats were measured using relevant ELISA kits (Abcam, Cambridge, UK). The procedures were carried out according to the manufacturer’s manuals.
Statistical analysis
The results were analysed using SPSS 17.0 software (IBM, Armonk, NY, USA). To test the difference between the means of multiple treatment groups, t-test or one-way ANOVA were used. Results were considered statistically significant if P<0.05. Data were shown as means ± standard error of the mean.
Results
Abnormal phlegmatic syndrome and ED model rats have disordered biological parameters
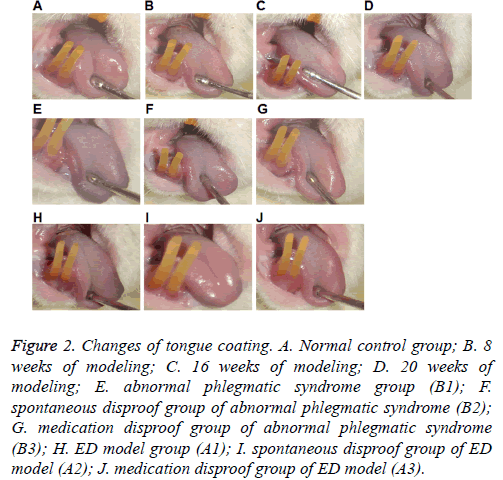
To assess abnormal phlegmatic syndrome and ED rat model, biological parameters of the rats were first studied. No treatment-related mortality in rats fed with 30% spinach and coriander diet in a wet-cold condition was observed within 20 weeks. The abnormal phlegmatic syndrome and ED model rats showed inactivity, clustering, slow movement, messy and dull hair, clear and colorless urine, loose and soft stool, dark tongue, greasy tongue coating and drollness (Figure 2). Compared with normal control group, abnormal phlegmatic syndrome and ED model rats showed increased food consumption, urine production, and stool production, as well as decreased body weight and water consumption (P<0.05) (Table 1). The results suggest that abnormal phlegmatic syndrome and ED model rats have disordered biological parameters.
Figure 2: Changes of tongue coating. A. Normal control group; B. 8 weeks of modeling; C. 16 weeks of modeling; D. 20 weeks of modeling; E. abnormal phlegmatic syndrome group (B1); F. spontaneous disproof group of abnormal phlegmatic syndrome (B2); G. medication disproof group of abnormal phlegmatic syndrome (B3); H. ED model group (A1); I. spontaneous disproof group of ED model (A2); J. medication disproof group of ED model (A3).
| Items | Control group | Model group |
|---|---|---|
| Number of rats | 10 | 90 |
| Body weight (g) | 662.57 ± 11.51 | 528.70 ± 14.78* |
| Food consumption (g/100 g body weight) | 7.71 ± 0.42 | 10.25 ± 0.57* |
| Water consumption (g/100 g body weight) | 10.40 ± 0.29 | 8.91 ± 0.70* |
| Urine production (ml/100 g body weight) with in 24 h | 4.37 ± 0.30 | 10.12 ± 0.54* |
| Stool production (g/100 g body weight) within 24 h | 2.43 ± 0.20 | 3.33 ± 0.64* |
Note: Values are presented as means ± standard error. *P<0.05 compared with normal control group. Body weight, food consumption, water consumption, urine production and stool production are of equal importance in Uygur medicine.
Table 1. Biological parameters of abnormal phlegmatic syndrome and ED rat model induced by Uygur medicine at week 20 of model establishment.
Sexual behavior of abnormal phlegmatic syndrome and ED rat model induced by wet-cold diet and living condition is inhibited significantly
To understand more about abnormal phlegmatic syndrome and ED rat model, sexual behaviors of the rats were further investigated. After APO (85 μg/kg) injection, 30 min sexual behavior test showed that abnormal phlegmatic syndrome and ED model group had increased latency of mounting, erectile, insertion, and ejaculation, as well as decreased number of mounting, erectile, insertion, and ejaculation, compared with normal control group (P<0.05) (Table 2). The results indicate that sexual behavior of abnormal phlegmatic syndrome and ED rat model induced by wet-cold diet and living condition is inhibited significantly.
| Group | n | Mounting latency (sec) | Mounting number | Erection latency (sec) | Erection number | Insertion latency (sec) | Insertion number | Ejaculation latency (sec) | Ejaculation number |
|---|---|---|---|---|---|---|---|---|---|
| Control | 10 | 414.70 ± 202.25 | 32.60 ± 7.52 | 479.3 ± 143.1 | 1.90 ± 0.74 | 553.20 ± 198.10 | 23.90 ± 8.10 | 666.70 ± 230.79 | 14.50 ± 3.95 |
| Model | 90 | 879.76 ± 567.50* (p=0.013) | 14.49 ± 12.30* (p=0.027) | 1381.43 ± 536.81* (p=0.003) | 0.49 ± 0.57* (p=0.029) | 1420.43 ± 568.84* (p=0.007) | 4.95 ± 8.60* (p=0.042) | 1433.60 ± 549.01* (p=0.013) | 2.63 ± 4.61* |
Note: Values are presented as means ± standard error. *P<0.05 compared with normal control group.
Table 2. Sexual behavior test of abnormal phlegmatic syndrome and ED rat model induced by Uygur medicine at week 20 of model establishment.
Abnormal phlegmatic syndrome and ED rat model induced by Uyghur medicine can mimic the symptom of abnormal phlegmatic syndrome and ED patients, and the model is reversible and relatively stable
To further evaluate the abnormal phlegmatic syndrome and ED rat model, we divided the model rats into 6 groups based on erection test. After 2 weeks of spontaneous disproof treatment and medication disproof treatment, compared with normal control group, decreased body weight and water consumption, and increased food consumption and urine production were observed in A1, A2, A3, B1, B2, and B3 groups (P<0.05), while increased stool production was detected in A1, A2, A3, and B1 groups (P<0.05). Compared with A1 group, increased weight and water consumption were observed in A3 group (P<0.05), while decreased food consumption, urine and stool production were observed in A2 and A3 groups (P<0.05). Compared with A2 group, increased body weight and water consumption, and decreased food consumption, urine production and stool production were observed in A3 group (P<0.05). Compared with B1 group, increased body weight and water consumption, and decreased food consumption, urine production and stool production were observed in B2 and B3 groups (P<0.05). Compared with B2 group, increased body weight and water consumption, and decreased food consumption, urine production and stool production were observed in B3 group, but there was no statistical significance (P>0.05) (Table 3). The results suggest that the abnormal phlegmatic syndrome and ED rat model induced by Uyghur medicine can mimic the symptom of abnormal phlegmatic syndrome and ED patients, and the model is reversible and relatively stable
| Group | n | Body weight (g) | Food consumption (g/100 g body weight) | Water consumption (g/100 g body weight) | Urine volume (ml/100 g body weight) | Stool production (g/100 g body weight) |
|---|---|---|---|---|---|---|
| Pre-disproof period | ||||||
| Normal control | 10 | 662.57 ± 11.51 | 7.71 ± 0.42 | 10.40 ± 0.29 | 4.37 ± 0.30 | 2.43 ± 0.20 |
| A1 | 13 | 479.63 ± 13.52* | 10.21 ± 0.52* | 8.68 ± 0.65* | 9.92 ± 0.59* | 3.69 ± 0.58* |
| A2 | 18 | 536.49 ± 15.41* | 10.89 ± 0.62* | 8.51 ± 0.62* | 10.39 ± 0.54* | 3.97 ± 0.64* |
| A3 | 18 | 551.33 ± 14.91* | 9.96 ± 0.58* | 9.42 ± 0.73* | 11.18 ± 0.58* | 3.64 ± 0.63* |
| B1 | 11 | 523.04 ± 15.09* | 9.81 ± 0.61* | 9.74 ± 0.69* | 9.73 ± 0.49* | 2.86 ± 0.61* |
| B2 | 15 | 583.32 ± 15.69* | 10.25 ± 0.53* | 8.24 ± 0.75* | 9.31 ± 0.50* | 2.69 ± 0.69* |
| B3 | 15 | 498.36 ± 14.05* | 10.39 ± 0.55* | 8.89 ± 0.72* | 10.21 ± 0.52* | 3.15 ± 0.67* |
| Post-disproof period | ||||||
| Normal control | 10 | 678.59 ± 12.92 | 7.66 ± 0.30 | 10.45 ± 0.25 | 5.09 ± 0.19 | 2.85 ± 0.23 |
| A1 | 13 | 549.11 ± 15.04* | 9.99 ± 0.28* | 8.44 ± 0.40* | 10.02 ± 0.32* | 3.19 ± 0.19* |
| A2 | 18 | 550.82 ± 5.06* | 9.37 ± 0.38*& | 8.74 ± 0.38& | 9.05 ± 0.46*& | 3.01 ± 0.36* |
| A3 | 18 | 540.45 ± 6.58*&$ | 9.13 ± 0.60*&$ | 9.25 ± 0.40*&$ | 8.40 ± 0.37*&$ | 2.56 ± 0.30*&$ |
| B1 | 11 | 539.20 ± 3.21* | 10.30 ± 0.26* | 8.78 ± 0.56* | 9.74 ± 0.37* | 3.40 ± 0.45* |
| B2 | 15 | 639.53 ± 9.34*# | 9.19 ± 0.30*# | 9.89 ± 0.65*# | 8.77 ± 0.62*# | 2.65 ± 0.30# |
| B3 | 15 | 667.73 ± 11.00*# | 8.91 ± 0.38*# | 10.00 ± 0.32*# | 8.60 ± 0.52*# | 2.56 ± 0.32# |
Note: Abnormal phlegmatic syndrome model rats were randomly divided into 6 different groups: abnormal phlegmatic syndrome group (B1, normal APO erectile test, contagiously lived in the same experimental condition); spontaneous disproof group of abnormal phlegmatic syndrome (B2, normal APO erectile test, moved to normal condition with normal diet); medication disproof group of abnormal phlegmatic syndrome (B3, normal APO erectile test, moved to normal condition with normal diet plus anti-ED drug); ED model group (A1, abnormal APO erectile test, lived in the same experimental condition); spontaneous disproof group of ED model (A2, abnormal APO erectile test, moved to normal condition with normal diet); medication disproof group of ED model (A3, abnormal APO erectile test, moved to normal condition with normal diet plus anti-ED drug). Values are presented as means ± standard error. *P<0.05 compared with normal control group. &P<0.05 compared with A1 group. $P<0.05 compared with A2 group. #P<0.05 compared with B1 group. Abnormal phlegmatic syndrome group (B1); spontaneous disproof group of abnormal phlegmatic syndrome (B2); medication disproof group of abnormal phlegmatic syndrome (B3); ED model group (A1); spontaneous disproof group of ED model (A2); medication disproof group of ED model (A3).
Table 3. Biological parameters of abnormal phlegmatic syndrome and ED rat model before and after disproof treatment.
Disproof treatment improves sexual behavior of abnormal phlegmatic syndrome and ED rat model, especially for that of abnormal phlegmatic syndrome group
To investigate the effects of two disproof treatments on sexual behavior of abnormal phlegmatic syndrome and ED model rats, sexual behavior test was performed on the rats. Compared with normal control group, increased latency of mounting, erection, insertion, and ejaculation, and decreased numbers of mounting, erection, insertion, and ejaculation were observed in A1, A2, A3, B1, B2, and B3 groups (P<0.05). Compared with A1 group, decreased latency of erection, insertion, and ejaculation, and increased numbers of erection, insertion, and ejaculation were observed in A3 group (P<0.05). Compared with A2 group, decreased latency of erection and increased number of erection were observed in A3 group (P<0.05).
Compared with B1 group, decreased latency of erection and increased number of mounting, erection, and ejaculation were observed in B2 group (P<0.05), while decreased latency of erection, insertion, and ejaculation, and increased number of mounting, erection, insertion, and ejaculation were observed in B3 group (P<0.05) (Table 4). The results indicate that disproof treatment improves sexual behavior of abnormal phlegmatic syndrome and ED rat model, especially for that of abnormal phlegmatic syndrome group.
| Group | n | Mounting latency | Mounting number | Erection latency (seconds) | Erection number | Insertion latency | Insertion number | Ejaculation latency | Ejaculation number |
|---|---|---|---|---|---|---|---|---|---|
| Normal control | 10 | 426.60 ± 143.59 | 29.20 ± 9.55 | 547.00 ± 208.93 | 1.80 ± 0.63 | 512.60 ± 181.28 | 21.90 ± 7.49 | 559.20 ± 164.11 | 11.70 ± 4.42 |
| A1 | 13 | 1101.08 ± 770.89* | 6.50 ± 7.40* | 1800.00 ± 0.00* | 0.00 ± 0.00* | 1800.00 ± 0.00* | 0.00 ± 0.00* | 1800.00 ± 0.00* | 0.00 ± 0.00* |
| A2 | 18 | 955.25 ± 555.17* | 10.83 ± 9.31* | 1460.83 ± 457.56* | 0.50 ± 0.67* | 1491.75 ± 489.38* | 4.08 ± 7.17* | 1511.83 ± 468.88* | 2.42 ± 4.60* |
| A3 | 18 | 890.5 ± 737.89 | 13.75 ± 16.00* | 680.00 ± 584.80&$ | 1.67 ± 1.23&$ | 1087.50 ± 715.86*& | 9.75 ± 12.65*& | 1098.17 ± 703.53*&$ | 5.92 ± 7.90*& |
| B1 | 11 | 910.55 ± 608.40* | 9.73 ± 11.55* | 713.45 ± 581.79 | 1.36 ± 0.92 | 1116.27 ± 670.31* | 6.55 ± 9.07* | 1149.64 ± 645.62* | 3.73 ± 5.64* |
| B2 | 15 | 465.27 ± 222.49 | 20.82 ± 10.99# | 887.36 ± 518.29# | 1.45 ± 0.69# | 732.27 ± 579.27 | 14.18 ± 10.53 | 787.91 ± 554.4 | 10.45 ± 8.51# |
| B3 | 15 | 480.00 ± 231.79 | 24.80 ± 10.57# | 680.00 ± 357.98# | 1.60 ± 1.07# | 589.60 ± 234.21# | 18.50 ± 9.76# | 600.70 ± 236.32# | 12.60 ± 6.60# |
Note: Values are presented as means ± standard error. *P<0.05 compared with normal control group. &P<0.05 compared with A1 group. $P<0.05 compared with A2 group. #P<0.05 compared with B1 group.
Table 4. Sexual behavior of abnormal phlegmatic syndrome and ED rat model after disproof treatment.
Decreased testosterone and increased estradiol, luteinizing hormone and prolactin are observed in abnormal phlegmatic syndrome and ED groups, but disproof treatment reverses these changes
To test the levels of testosterone, estradiol, luteinizing hormone, follicle stimulating hormone and prolactin in rats, ELISA was performed. The data showed that testosterone levels in A1, A2, and B1 groups were significantly lower than that of normal control group. The level of testosterone in A3 group was significantly higher than those in A1 and A2 groups, and no significant difference was observed between A1 and A2. Testosterone levels in B2 and B3 groups were significantly higher than that in B1 group (P<0.05). Compared with normal control group, the level of estradiol in A1 group was significantly higher (P<0.05). Compared with normal control group, luteinizing hormone levels in A1 and B3 groups were significantly higher (P<0.05). In addition, the levels of follicle stimulating hormone in the 6 groups were not significantly different from that of normal control group (P>0.05). Compared with normal control group, the level of PRL was increased in A1 group; compared with A1 group, the level of PRL was decreased in A3 group (P<0.05) (Table 5). These results suggest that decreased testosterone and increased estradiol, luteinizing hormone and prolactin are observed in abnormal phlegmatic syndrome and ED groups, but disproof treatment reverses these changes.
| Group | n | T (ng/ml) | E2 (mIU/ml) | LH (mIU /ml) | FSH (mIU /ml) | PRL (mIU /ml) |
|---|---|---|---|---|---|---|
| Normal control | 10 | 3.51 ± 1.14 | 4121.51 ± 1510.96 | 2.07 ± 1.35 | 2.50 ± 1.12 | 80.28 ± 25.15 |
| A1 | 13 | 0.93 ± 0.53* | 5153.90 ± 962.97* | 3.76 ± 1.14* | 2.41 ± 0.79 | 111.83 ± 36.15* |
| A2 | 18 | 1.10 ± 0.46* | 5006.01 ± 830.32 | 3.27 ± 1.82 | 2.75 ± 1.15 | 100.86 ± 35.03 |
| A3 | 18 | 2.83 ± 1.39&$ | 4149.69 ± 729.87& | 2.58 ± 1.42 | 2.84 ± 0.85 | 81.53 ± 22.22& |
| B1 | 11 | 1.33 ± 0.50* | 4904.50 ± 841.56 | 2.90 ± 2.06 | 2.78 ± 0.71 | 98.80 ± 32.98 |
| B2 | 15 | 2.51 ± 1.66# | 4373.07 ± 790.97 | 3.09 ± 2.09 | 2.66 ± 0.49 | 92.12 ± 22.35 |
| B3 | 15 | 3.54 ± 1.96# | 4424.16 ± 1100.78 | 2.65 ± 1.90 | 2.77 ± 0.90 | 81.20 ± 24.76 |
Note: Values are presented as means ± standard error. *P<0.05 compared with normal control group. &P<0.05 compared with A1 group. $P<0.05 compared with A2 group. #P<0.05 compared with B1 group. T: Testosterone; LH: Luteinizing Hormones; FSH: Follicle Stimulating Hormone; E2: Estradiol; PRL: Prolactin.
Table 5. Sexual hormone levels in different groups of rats after disproof treatment.
Discussion
Normal sexual function has been described as a biopsychosocial process that involves the coordination of psychological, endocrine, vascular, and neurological systems. ED is classified as psychogenic, organic or mixed psychogenic and organic. It is a common, yet debilitating, condition affecting both men and women at some point in their lives and preventing them from experiencing satisfaction from the sexual activity [15]. Findings from epidemiological studies confirm that aging, type 2 diabetes mellitus, sedentary lifestyle, smoking, alcohol or drug misuse, sleep disorders, obesity, and metabolic syndromes are all associated with ED [16,17]. Substantial advances have been achieved in the understanding of the pathophysiology of ED and led to the development of successful oral therapies using PDE5 inhibitors. However, oral PDE5 inhibitors have limitations even when being combined with cholesterol-lowering drug atorvastatin, PDE5 inhibitor sildenafil is unable to fully restore erectile function [7]. Uyghur medicine has obvious effect to alleviate ED, but no perfect ED animal model exists to further study its roles and mechanism and hence, limiting the development of Uyghur medicine. Therefore, the present study aims to establish and provide a desired scientific ED rat model on the basis of Uyghur traditional medicine.
Abnormal phlegmatic syndrome with ED is a systemic disease rather than an organ disease that is usually caused by wet-cold diet and environment, or less movement. The body fluid is wetcold, and affects the function of brain, heart, liver and kidney, finally leading to ED. In clinic, these patients have face, eye and tongue with white color, physical puffiness, drowsiness, drooling, urorrhagia, clear urine and impotence. In the present study, we have created an abnormal phlegmatic syndrome with ED rat model using wet-cold diet and living condition based on the theory of Uyghur medicine. The model rats have shown inactivity, dull hair, clear urine, loose and soft stool, dark tongue, greasy tongue coating, decreased body weight and water consumption, and increased food consumption, urine production, and stool production. These biological parameters are consistent with abnormal phlegmatic syndrome with ED patients diagnosed by Uyghur medicsine. Sexual behavior test has shown increased mounting, erectile, insertion, and ejaculation latency, and decreased mounting, erectile, insertion, and ejaculation numbers in abnormal phlegmatic syndrome group, while these changes are more severe in ED rats. The results indicate that ED rat model establishment successfully mimic ED process in human. After removing the wet-cold diet and environment, these changes in spontaneous disproof group are alleviated mildly, indicating that model establishment requires wet-cold diet and environment, and the model is stable. After being bred in normal condition and treated with anti-ED drug the biological parameters, sexual behavior and sexual hormones of ED model rats were reversed significantly, suggesting that the abnormal phlegmatic syndrome and ED rat model induced by wet-cold diet and environment is reversible, scientific and feasible. To summarize, we conclude that the abnormal phlegmatic syndrome and ED rat model induced by wet-cold diet and environment mimics the development of abnormal phlegmatic syndrome with ED and shows obvious changes consistent with clinic patients. Moreover, the model can be reversed after treatment. Therefore, this model is a desired, scientific and stable rat model of abnormal phlegmatic syndrome with ED.
Acknowledgements
The project was supported by grants from the National Natural Science Foundation of China (Nos. 81160470 and 81260581).
Disclosure of Conflict of Interest
None
References
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013; 381: 153-165.
- Fahmy UA. Nanoethosomal transdermal delivery of vardenafil for treatment of erectile dysfunction: optimization, characterization, and in vivo evaluation. Drug Des Devel Ther 2015; 9: 6129-6137.
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999; 84: 50-56.
- Oboh G, Ademiluyi AO, Ademosun AO, Olasehinde TA, Oyeleye SI, Boligon AA, Athayde ML. Phenolic extract from Moringa oleifera leaves inhibits key enzymes linked to erectile dysfunction and oxidative stress in rats penile tissues. Biochem Res Int 2015; 2015: 175950.
- Lin CS, Xin ZC, Wang Z, Deng C, Huang YC. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev 2012; 21: 343-351.
- Tang WH, Zhuang XJ, Ma LL, Hong K, Zhao LM, Liu DF, Mao JM, Zhang HL, Jiang H. Effect of sildenafil on erectile dysfunction and improvement in the quality of sexual life in China: a multi-center study. Int J Clin Exp Med 2015; 8: 11539-11543.
- Dadkhah F, Safarinejad MR, Asgari MA, Hosseini SY, Lashay A. Atorvastatin improves the response to sildenafil in hypercholesterolemic men with erectile dysfunction not initially responsive to sildenafil. Int J Impot Res 2010; 22: 51-60.
- Miner M, Billups KL. Erectile dysfunction and dyslipidemia: relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med 2008; 5: 1066-1078.
- Zhuyan L, Maimaitizunong M, Liu F, Maowulan M, Zhang P, Abudureyimujiang R, Chen S. The effects of Yimusake on penis tissue morphological changes of rat models with abnormal balgam syndrome and impotence disease induced by Uyghur medicine. Chin J Androl 2015; 29: 9-14.
- Upur H, Dubrovin D, Amat N, Liu W, Moore N, Bauer R. Greco-Arab- Uighur medicine. USA Acupress 2013.
- Upur H, Yusufu A, Aimaiti N. New interpretations of abnormal savda theory of traditional Uighur medicine. China, Urumqi Xinjiang Peoples Press 2009.
- Upur H, Chen Y, Kamilijiang M, Deng W, Sulaiman X, Aizezi R, Wu X, Tulake W, Abudula A. Identification of plasma protein markers common to patients with malignant tumour and Abnormal Savda in Uighur medicine: a prospective clinical study. BMC Complement Altern Med 2015; 15: 9.
- Ailiaji kuerbanniyazimiqi. Uyghur medicine therapy on erectile dysfunction. Urumqi Xinjiang Peoples Health Publ H 2006: 31-99.
- Alipu T, Gulisitan A. Diet therapy of Uyghur medicine on male hypogonadism. J Med Pharm Chin Minorities 2006; 12: 72.
- Mourikis I, Antoniou M, Matsouka E, Vousoura E, Tzavara C, Ekizoglou C, Papadimitriou GN, Vaidakis N, Zervas IM. Anxiety and depression among Greek men with primary erectile dysfunction and premature ejaculation. Ann Gen Psychiatry 2015; 14: 34.
- El Saghier EO, Shebl SE, Fawzy OA, Eltayeb IM, Bekhet LM. Androgen deficiency and erectile dysfunction in patients with type 2 diabetes. Clin Med Insights Endocrinol Diabetes 2015; 8: 55-62.
- Huang YC, Chin CC, Chen CS, Shindel AW, Ho DR, Lin CS, Shi CS. Chronic cigarette smoking impairs erectile function through increased oxidative stress and apoptosis, decreased nNOS, endothelial and smooth muscle contents in a rat model. PLoS One 2015; 10: 0140728.