Research Article - Biomedical Research (2017) Volume 28, Issue 4
Randomized phase II study of erlotinib as first-line or second-line therapy for EGFR mutation-positive advanced lung adenocarcinoma patients
Wei Zhao1, Xingchen Liu1, Qing Tian1, Yan Chang2, Zhen Yang1 and Liangan Chen1*1Department of Respiratory Medicine, Chinese PLA General Hospital, Beijing, China
2Department of Respiratory Medicine, the General Hospital of the PLA Rocket Force, Beijing, China
- *Corresponding Author:
- Liangan Chen
Department of Respiratory Medicine
Chinese PLA General Hospital, China
Accepted on October 5, 2016
Abstract
Epidermal Growth Factor Receptor (EGFR) mutation in patients with Non-Small Cell Lung Cancer (NSCLC) in Asian population could benefit in survival to Tyrosine Kinase Inhibitor (TKI) treatment. Standard chemotherapy was the mainstay for advanced NSCLC. In this randomized, single center, phase 2 studies, we investigated erlotinib as first-line therapy plus docetaxel and cisplatin as second-line therapy compared to docetaxel and cisplatin as first-line therapy plus erlotinib as second-line therapy in treat naïve patients with advanced lung adenocarcinoma. 81 patients were randomized to receive erlotinib+chemotherapy (E+C group, n=43) and chemotherapy+erlotinib (C+E group, n=38). The primary objective was Overall Survival (OS) and secondary objective was objective response rate. The data showed the median OS in E+C group and C+E group were 23.7 months and 24.2 months, respectively (p=0.652). The objective response rate differed in the two groups (p<0.001). Our results indicated that sequential usage of erlotinib plus chemotherapy and chemotherapy plus erlotinib did not showed difference in OS.
Keywords
Erlotinib, EGFR, Lung adenocarcinoma, RCT.
Introduction
Lung cancer is the most commonly diagnosed cancer type and the leading cause of cancer-related deaths around the world [1]. Non-Small Cell Lung Cancer (NSCLC) accounts for the majority of lung cancers. Lung adenocarcinoma is a subtype of NSCLC and is often the main subject to Tyrosine Kinase Inhibitor (TKI) treatment of the Epidermal Growth Factor Receptor (EGFR) [2]. Several FDA-approved TKIs including Gefitinib, Erlotinib and Afatinib showed inspiring effects to patients with NSCLC and were recommended as the first-line or second-line treatment [3-8]. Patients with EGFR mutations are usually highly sensitive to EGFR Tyrosine Kinase Inhibitor (EGFR-TKI) therapy [9-13]. Sensitizing EGFR mutations were found in about 10% of Caucasian NSCLC patients, however, this proportion was up to about 50% in Asian population [14]. Therefore, Asian population with EGFR exon 19 deletions or exon 21 L858R mutations could benefit most from EGFR-TKIs theoretically.
Accumulating evidence has shown erlotinib could prolong the survival time of mutation-positive NSCLC [9,12,15,16], however, these studies did not investigated the comparison of sequential usage of TKI and standard chemotherapy. In the current randomized, phase II study, we explored the treatment regimen of erlotinib as first-line therapy plus docetaxel and cisplatin as second-line therapy compared to docetaxel and cisplatin as first-line therapy plus erlotinib as second-line therapy in treatment of naïve EGFR mutation-positive lung adenocarcinoma patients with stage III or IV.
Materials and Methods
Eligibility
Patients with stages III-IV (AJCC 6th edition) lung adenocarcinoma who did not receive any treatment were considered for enrolment. Patients also had to be aged >18 years; have an Eastern Cooperative Oncology Group (ECOG) performance Status (PS) of 0-2; have measurable disease by the Response Evaluation Criteria (RECIST; version 1.1); have expected life expectancy of more than 12 weeks and have adequate organ function.
The exclusion criteria included previous treatment for NSCLC; patients with wild-type EGFR; HIV infection; with component of small cell lung cancer; pregnancy; had a myocardial infarction within 6 months. This trial was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice.
The Ethics Committees of the Chinese PLA General Hospital reviewed and approved the trial protocol. The trail was registered in www.clinicaltrials.gov (NCT01131429).
Study design and treatment
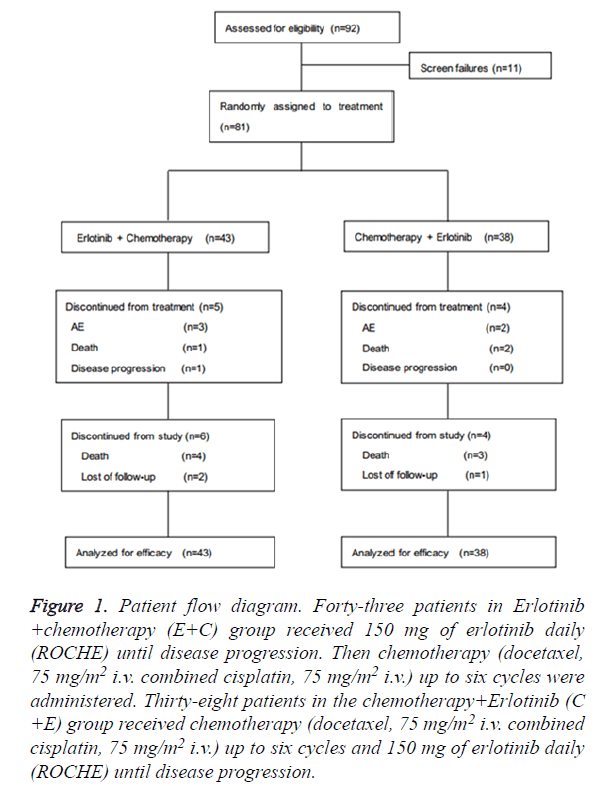
This was a randomized, single center; phase 2 study erlotinib as first-line therapy plus docetaxel and cisplatin as second-line therapy compared to docetaxel and cisplatin as first-line therapy plus erlotinib as second-line therapy in treat naïve patients with advanced lung adenocarcinoma. Eligible patients were randomized before induction therapy in a 1:1 ratio to receive treatment (Figure 1).
Treatment
Patients in the Erlotinib+chemotherapy (E+C) group received 150 mg of erlotinib daily (ROCHE) until disease progression. Then chemotherapy (docetaxel, 75 mg/m2 i.v. combined cisplatin, 75 mg/m2 i.v.) up to six cycles were administered. Patients in the chemotherapy+Erlotinib (C+E) group received chemotherapy (docetaxel, 75 mg/m2 i.v. combined cisplatin, 75 mg/m2 i.v.) up to six cycles and 150 mg of erlotinib daily (ROCHE) until disease progression.
Assessments
The primary objective was to assess OS in patients in each group. Secondary objectives included the radiographic objective response rate (ORR; evaluated every 2 cycles) according to RECIST (version 1.1).
Statistical analysis
The primary cut-off date for OS data was May 2015. OS were estimated by the Kaplan-Meier method. The difference between the two groups was assessed by means of log-rank tests. Chi-square tests were used to compare categorical data. The probability of OS was estimated using the Kaplan-Meier method, and survival curves were compared using a log-rank test. Hazard Ratios (HRs) and 95% Confidence Intervals (CIs) were calculated using a Cox proportional hazards analysis. Two-sided P values of<0.05 were considered as statistically significant.
All statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
A total of 81 patients were randomized to receive either erlotinib and chemotherapy (43 patients) or chemotherapy and erlotinib (38 patients) at Chinese PLA General Hospital (Figure 1). The median follow-up was 11.7 months. The baseline characteristics including age, sex, ECOG PS, smoking history and EGFR mutations were well balanced between the two treatment arms (Table 1).
Figure 1. Patient flow diagram. Forty-three patients in Erlotinib +chemotherapy (E+C) group received 150 mg of erlotinib daily (ROCHE) until disease progression. Then chemotherapy (docetaxel, 75 mg/m2 i.v. combined cisplatin, 75 mg/m2 i.v.) up to six cycles were administered. Thirty-eight patients in the chemotherapy+Erlotinib (C +E) group received chemotherapy (docetaxel, 75 mg/m2 i.v. combined cisplatin, 75 mg/m2 i.v.) up to six cycles and 150 mg of erlotinib daily (ROCHE) until disease progression.
| Characteristics | Erlotinib+ Chemotherapy (n=43) |
Chemotherapy+ Erlotinib (n=38) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Gender | ||||
| Male | 9 | 20.9 | 14 | 36.8 |
| Female | 34 | 79.1 | 24 | 63.2 |
| Age, years | ||||
| Median | 59 | 57 | ||
| Range | 35-78 | 34-75 | ||
| Smoking status | ||||
| Never smoker | 38 | 88.4 | 30 | 78.9 |
| Smoker | 5 | 11.6 | 8 | 21.1 |
| Family history of cancer | ||||
| No | 31 | 72.1 | 26 | 68.4 |
| Yes | 12 | 27.9 | 12 | 31.6 |
| Adenocarcinoma stage | ||||
| IIIB | 1 | 2.3 | 2 | 5.3 |
| IV | 42 | 97.7 | 36 | 94.7 |
| ECOG PS | ||||
| 0 | 0 | 0 | 0 | 0 |
| 1 | 35 | 81.4 | 33 | 86.8 |
| 2 | 8 | 18.6 | 5 | 13.2 |
| Differentiation | ||||
| Well | 4 | 9.3 | 2 | 5.3 |
| Moderate | 6 | 14 | 10 | 26.3 |
| Poor | 11 | 25.6 | 16 | 42.1 |
| Unknown | 22 | 51.1 | 10 | 26.3 |
| EGFR mutation | ||||
| Exon 19 deletion | 24 | 55.8 | 23 | 60.5 |
| Exon 21 L858R mutation | 19 | 44.2 | 15 | 39.5 |
Table 1. Baseline patient characteristics in both treatment groups.
Efficacy
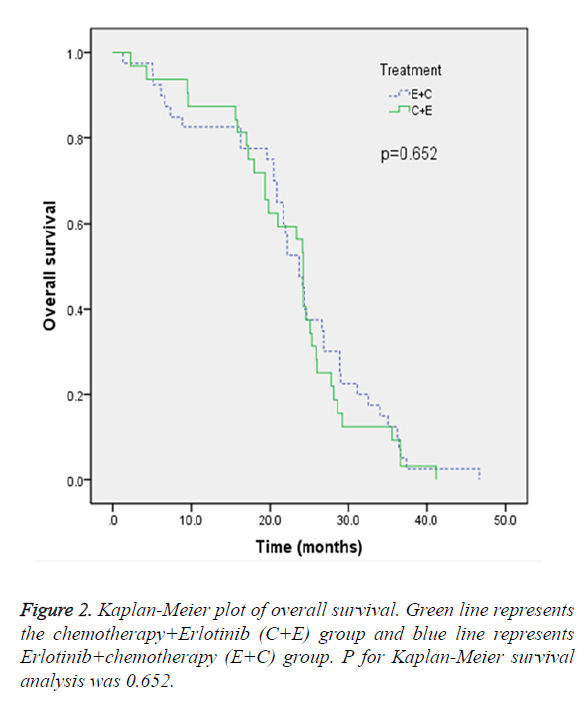
In the ITT population, the median OS in E+C group and C+E group were 23.7 months and 24.2 months, respectively. The difference did not reach statistical significance (p=0.652) (Figure 2). The objective response rate after first or second line treatment evaluated by RECIST (version 1.1) was demonstrated in Table 2. In the E+C group, after erlotinib as first-line treatment, 55.8% of the patients had PR and 44.2% of the patients had SD, and after chemotherapy as second-line therapy, 17.9% of the patients had PR, 76.9% of the patients had SD and 5.1% of the patients had others.
| Variable | Erlotinib + Chemotherapy (n=43) | Chemotherapy + Erlotinib (n=38) | P value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| After 1st line treatment | <0.001 | ||||
| CR | 0 | 0 | 0 | 0 | |
| PR | 24 | 55.8 | 4 | 10.5 | |
| SD | 19 | 44.2 | 22 | 57.9 | |
| PD | 0 | 0 | 3 | 7.9 | |
| Others | 0 | 0 | 9 | 23.7 | |
| After 2nd line treatment | n=39 | n=30 | |||
| CR | 0 | 0 | 0 | 0 | <0.001 |
| PR | 7 | 17.9 | 19 | 63.3 | |
| SD | 30 | 76.9 | 11 | 36.7 | |
| PD | 0 | 0 | 0 | 0 | |
| Others | 2 | 5.1 | 0 | 0 | |
Table 2. Objective response rate for each treatment group.
In the C+E group, after chemotherapy as first-line therapy, 10.5% of the patients had PR, 57.9% of the patients had SD, 7.9% of the patients had PD and 23.7% of the patients had others and after erlotinib as second-line therapy, 63.3% of the patients had PR and 36.7% of the patients had SD.
Discussion
EGFR mutation was an important biomarker in NSCLC with regard to responsiveness to EGFR TKIs [12,13,15-20]. To our knowledge, the current study was the first study to prospectively investigate erlotinib as first-line combined stand chemotherapy as second-line therapy versus chemotherapy as first-line therapy combined erlotinib as second-line therapy in Chinese population. Our results demonstrated that the two treatment regimen did not differ in OS (p=0.652). The objective response rate analyses showed that PR rate was higher in E+C group after first-line treatment, whereas, PR rate was higher in C+E group after second-line treatment. These data further confirm that the erlotinib regimen would be suitable for Asian patients with EGFR mutation.
Recently, ENSURE study investigated first-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer patients. Their results showed first-line erlotinib provides a statistically significant improvement in PFS versus GP [21]. The PFS benefit of erlotinib in EGFR mutation-positive patients was confirmed by OPTIMAL trail [22]. Our study did not investigated PFS as a monitored parameter because the patients recruited had III or IV disease. Notably, in ENSURE study, the data showed that OS was not significantly different between the two arms, which were in line with our results. Our data showed the objective response rate differed between the two treatment groups, which was possible due to the different treatment sequence.
There were several limitations in our study. First, the sample size was relatively small, which was due to limited resources and a difficult search for grant support. Second, the uses of the more traditional cisplatin/docetaxel regimen as a comparator instead of more novel combinations such as cisplatin/ pemetrexed are still needed.
In conclusion, the results of our study showed the sequential usage of erlotinib as first-line therapy plus docetaxel and cisplatin as second-line therapy compared to docetaxel and cisplatin as first-line therapy plus erlotinib as second-line therapy in treat naive patients with advanced lung adenocarcinoma did not show prolonged OS. In addition, the objective response rate varied between the two groups.
Acknowledgements
We thank the patients who participated in this trial.
Disclosure of Conflict of Interest
None.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87-108.
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380-2388.
- Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, DAmico TA, Demmy TL, Dilling TJ, Govindan R, Grannis FW Jr., Horn L, Jahan TM, Komaki R, Kris MG, Krug LM, Lackner RP, Lanuti M, Lilenbaum R, Lin J, Loo BW Jr., Martins R, Otterson GA, Patel JD, Pisters KM, Reckamp K, Riely GJ, Rohren E, Schild S, Shapiro TA, Swanson SJ, Tauer K, Yang SC, Gregory K. Non-small cell lung cancer. J Natl Compr Canc Netw 2015; 13: 515-524.
- Lee CK, Wu YL, Ding PN, Lord SJ, Inoue A, Zhou CC, Mitsudomi T, Rosell R, Pavlakis N, Links M, Gebski V, Gralla RJ, Yang JCH. Impact of Specific Epidermal Growth Factor Receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with egfr tyrosine kinase inhibitors versus chemotherapy in egfr-mutant lung cancer: A meta-analysis. J Clin Oncol 2015; 33: 1958-U1142.
- Burotto M, Manasanch EE, Wilkerson J, Fojo T. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 2015; 20: 400-410.
- Wu YL, Zhou CC, Hu CP, Feng JF, Lu S, Huang YC, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213-222.
- Yang JCH, Shih JY, Su WC, Hsia TC, Tsai CM, Ou SHI, Yu CJ, Chang GC, Ho CL, Sequist LV, Dudek AZ, Shahidi M, Cong XJ, Lorence RM, Yang PC, Miller VA. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 2012; 13: 539-548.
- Wang F, Wang LD, Li B, Sheng ZX. Gefitinib compared with systemic chemotherapy as first-line treatment for chemotherapy-naive patients with advanced non-small cell lung cancer: A meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 2012; 24: 396-401.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Munoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Grp Francais P. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239-246.
- Yang JCH, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou CC, Hu CP, OByrne K, Feng JF, Lu S, Huang YC, Geater SL, Lee KY, Tsai CM, Gorbunova V, Hirsh V, Bennouna J, Orlov S, Mok T, Boyer M, Su WC, Lee KH, Kato T, Massey D, Shahidi M, Zazulina V, Sequist LV. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141-151.
- Boeck S, Vehling-Kaiser U, Waldschmidt D, Kettner E, Marten A, Winkelmann C, Klein S, Kojouharoff G, Gauler T, Fischer von Weikersthal L, Clemens MR, Geissler M, Greten TF, Hegewisch-Becker S, Neugebauer S, Heinemann V. Erlotinib 150 mg daily plus chemotherapy in advanced pancreatic cancer: an interim safety analysis of a multicenter, randomized, cross-over phase III trial of the Arbeitsgemeinschaft Internistische Onkologie. Anticancer Drugs 2010; 21: 94-100.
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol 2007; 25: 1960-1966.
- Dragovich T, Huberman M, Von Hoff DD, Rowinsky EK, Nadler P, Wood D, Hamilton M, Hage G, Wolf J, Patnaik A. Erlotinib plus gemcitabine in patients with unresectable pancreatic cancer and other solid tumors: phase IB trial. Cancer Chemother Pharmacol 2007; 60: 295-303.
- Li C, Fang R, Sun Y, Han X, Li F. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 2011; 6: e28204.
- Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 2005; 353: 133-144.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353: 123-132.
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497-1500.
- Kawaguchi T, Ando M, Asami K, Okano Y, Fukuda M. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014; 32: 1902-1908.
- Heymach JV, Lockwood SJ, Herbst RS, Johnson BE, Ryan AJ. EGFR biomarkers predict benefit from vandetanib in combination with docetaxel in a randomized phase III study of second-line treatment of patients with advanced non-small cell lung cancer. Ann Oncol 2014; 25: 1941-1948.
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169-181.
- Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, Lu S, Cheng Y, Han B, Chen L, Huang C, Qin S, Zhu Y, Pan H, Liang H, Li E, Jiang G, How SH, Fernando MCL, Zhang Y, Xia F, Zuo Y. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883-1889.
- Zhou CC, Wu YL, Chen GY, Feng JF, Liu XQ, Wang CL, Zhang SC, Wang J, Zhou SW, Ren SX, Lu S, Zhang L, Hu CP, Hu CH, Luo Y, Chen L, Ye M, Huang JN, Zhi XY, Zhang YP, Xiu QY, Ma J, Zhang L, You CX. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735-742.