Research Article - Biomedical Research (2017) Volume 28, Issue 8
Randomized controlled trial of follow-up in pancreatic cancer patients treated by intermediate frequency ultrasound combined with opioid analgesic
Li Li1*, Hong-Liang Li2#, Xiao-Hui Wang3# and Yibin Xie4
1Qilu Hospital of Shandong University (Qingdao), No.758 Hefei Road, Qingdao, PR China
2Department of Critical Care Medicine, Peking University Third Hospital, Beijing, PR China
3Department of General Surgery, Xuan Wu Hospital, Capital Medical University, Beijing, PR China
4Department of Abdominal Surgical Oncology, Cancer Hospital and Institute, Chinese Academy of Medical Sciences and Peking Union Medical College, No.17 Panjiayuan Nanli, Chaoyang District, Beijing, PR China
#These authors contributed equally to this work
Accepted on December 30, 2016
Abstract
Objective: The objective is to explore the clinical efficacy and safety of intermediate frequency ultrasound combined with potent opioid analgesic in treating malignant pancreatic cancer.
Methods: 162 malignant pancreatic cancer patients admitted in our hospital during February, 2010- February, 2013 were selected. The patients were divided into the observation group and control group according to the random number table method with 81 patients in either group. The patients in both groups received opioid analgesic (morphine), and the patients in the observation group were also treated with intermediate frequency ultrasound (high intensity focused ultrasound). The patients were followed up for 2 years to observe the therapeutic efficacy, safety and survival situation.
Results: The clinical benefit rate (CBR) was 84.0% (68 cases) in the observation groups; the CBR was 40.7% (33 cases) in the control group. The CBR in the observation group was significantly higher than the control group (P<0.05). After treatment, the visual analogue score (VAS) and morphine dose in the observation group were both significantly decreased (P<0.05), however the morphine dose in the control group was not changed (P>0.05), the VAS in the control group was also decreased but not as much as the observation group (P<0.05). After treatment, the serum vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) were both significantly decreased in the observation group (P<0.05), but were not changed in the control group (P>0.05). The vital signs in the two groups were not obviously changed, and there was no adverse reaction such as skin burn or organ perforation. All the patients in the two groups were effectively followed up, the average follow up time was (23.2 ± 9.1) months. The median survival time (MST) was (6.4 ± 1.3) months in the observation group and (3.6 ± 0.8) months in the control group. The MST in the observation group was significantly higher than the control group (P<0.05).
Conclusion: Intermediate frequency ultrasound combined with opioid analgesic can effectively alleviate cancerous pain, decrease VEGF and HGF, and inhibit the progress of the cancer in malignant pancreatic cancer patients, which is a safe and effective therapeutic regimen. It is significant in prolonging the survival time of patients.
Keywords
Intermediate frequency ultrasound, Opioid analgesic, Pancreatic cancer, Random, Control, Follow up.
Introduction
Pancreatic cancer is a common malignant gastrointestinal cancer, the incidence rate of which is increasing year by year [1,2]. The early stage symptoms are not obvious, which can cause misdiagnosis and missed diagnosis; meanwhile, pancreatic cancer is highly invasive in the early stage, and many patients are already in the advanced stage once confirmed [3-5]. Over 80% patients have lost the chance to receive surgical treatment, thus appropriate and effective conservative treatment is the key to improve the survival quality of patients [4,6-8]. Some scholars suggest treating patients by routine supportive treatment combined with potent opioid analgesic, which can improve the cancerous pain and the life quality of patients [9-11]. In the recent years, novel non-invasive and non-radioactive therapies presented by high intensity focused intermediate frequency ultrasound have received extensive attention [12-14]. Thus, intermediate frequency ultrasound combined with morphine is expected to have good effects in treating malignant pancreatic cancer. In this study, a prospective randomized controlled analysis was conducted.
Materials and Methods
Inclusion and exclusion criteria
A total of 162 pancreatic cancer patients admitted in our hospital during February, 2010-February, 2013 were selected. Inclusion criteria: Confirmed as pancreatic cancer by pathological puncture biopsy and imageological examination; Not suitable for the surgical resection; predicted survival time ≥ 3 months; Patients and surrogates both understand and agree to this study and sign the conformed consent. Exclusion criteria: Complicated with severe heart, liver or kidney disease; Have psychological or neurological disease history; Received radiotherapy or chemotherapy previously before inclusion; Complicated with coagulation dysfunction or obvious bleeding tendency; The case data is not complete or lost to follow up.
Grouping
The patients were divided into the observation group and control group according to the random number table method with 81 patients in either group. The age in the observation group was 41-75 years, the average was (62.9 ± 8.5) years, the tumor volume was 2 cm × 2 cm × 2 cm-9 cm × 7 cm × 7 cm. The age in the control group was 42-75 years, the average was (61.8±8.9) years, the tumor volume was 2 cm × 3 cm × 2 cm-9 cm × 7 cm × 8 cm. The comparison of other general clinical data between two groups is shown in Table 1. The age, vital signs, tumor volume, gender, TNM stage, tumor location and complications between two groups were not statistically different (P>0.05), which were comparable.
| Clinical data | Observation group (n=81) | Control group (n=81) | ||
|---|---|---|---|---|
| Male (n=46) | Female (n=35) | Male (n=48) | Female (n=33) | |
| Age (years) | 62.2 ± 8.4 | 63.0 ± 8.5 | 62.1 ± 8.4 | 61.5 ± 8.5 |
| Heart rate (/min) | 92.8 ± 11.5 | 91.7 ± 11.3 | 92.5 ± 10.9 | 91.5 ± 10.8 |
| Systolic pressure (mmHg) | 126.2 ± 13.8 | 124.7 ± 12.6 | 125.9 ± 12.1 | 125.7 ± 11.8 |
| TNM stage | ||||
| Stage III | 24 (52.2) | 19 (54.3) | 24 (50.0) | 16 (48.5) |
| Stage IV | 22 (47.8) | 16 (45.7) | 24 (50.0) | 17 (51.5) |
| Tumor location | ||||
| Pancreas head | 8 (17.4) | 6 (17.1) | 7 (14.6) | 6 (18.2) |
| Pancreas body | 16 (34.8) | 8 (22.9) | 16 (33.3) | 9 (27.3) |
| Pancreas tail | 22 (47.8) | 17 (48.6) | 24 (50.0) | 16 (48.5) |
| Complications | ||||
| Liver metastasis | 6 (13.0) | 5 (14.3) | 7 (14.6) | 5 (15.2) |
| Jaundice | 3 (6.5) | 3 (8.6) | 4 (8.3) | 2 (6.1) |
Table 1. Comparison of general clinical data between two groups (n).
Therapeutic methods
Routine treatment: The patients in the both groups received routine treatment for malignant pancreatic cancer, including fluid infusion, nutrition support, electrolyte recovery and acidbase balance et al, and symptomatic treatment was applied according to the symptoms. The patients with liver metastasis received liver protection drug (bifendate), and the patients who had severe jaundice received percutaneous transhepatic cholangial drainage (PTCD) to reduce the jaundice.
Analgesia therapy: Based on the routine treatment, the patients in the both groups received potent opioid analgesic. The patients orally took morphine hydrochloride pill (Qinghai Pharmaceutical Factory Co. Ltd., Xining, China), 1-1.5 pill every time (20 mg-30 mg) for every 12 hours. The morphine dose was adjusted accordingly. The analgesic treatment was continued for 21 days.
Intermediate frequency ultrasound: The patients in the observation group also received intermediate frequency ultrasound treatment. The equipment was JC-200 high intensity focused intermediate frequency ultrasound machine (Chongqing Haifu Medical Technology Co. Ltd., Chongqing, China). Before the operation, the patients took oral laxative, the skin was degassed and degreased, and skin at the treating area was immersed in circulating degassed liquid. The parameters: the frequency was 0.8 MHz, the acoustical power was 250 W-350 W and the treatment time was 1500 s-3500 s. The treatment was continued for 30 min-50 min once every day. After the operation, routine cold compress therapy was applied at the treating area for 20 min-30 min to avoid burning. The treatment was continued for 21 days.
Observation indexes
Clinical benefit rate (CBR): The CBR was evaluated. Clinical benefit [15,16]: Compared with before treatment, visual analogue score (VAS) decreases by more than 50%, the morphine dose decreases by more than 50%, Kamofsky performance status (KPS) score increases by more than 20 points or the body weight increases by more than 7%. The patients who conform to 1 of the above persisting for more than 4 weeks are considered to have clinical benefit.
Pain situation: VASs in two groups were evaluated and compared before treatment, at 15 and 21 days after treatment. VAS criteria: 0 stands for no pain: 1-3 stands for mild annoying pain and no analgesic is needed; 4-6 stands for nagging, uncomfortable, troublesome pain which affects sleep and low dose analgesic is needed; 7-10 stands for intolerant severe pain and high dose of analgesic is needed. The morphine dose during treatment was also recorded.
Serum factors: The vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in two groups at different stages were detected to analyze the therapeutic mechanism of intermediate frequency ultrasound.
Safety: The blood pressure, respiration, liver and kidney function, blood and urine routine test were closely monitored, the adverse reaction was observed, and the intermediate frequency ultrasound related adverse reactions such as skin burn and organ perforation were also observed.
Follow up
The patients were followed up for 2 years to observe the survival situation.
Statistical analysis
All the data in this clinical study were analyzed by SPSS18.0. The numeration data were presented as n% and analyzed by • 2 test. The measurement data were presented as (• x±s) and analyzed by t test. The inspection level was set as α=0.05, and P<0.05 was considered as statistically different.
Results
CBR
The CBR in the observation group was 84.0% (68 cases); the CBR in the observation group was 40.7% (33 cases); The CBR in the observation group was significantly higher than the control group (P<0.05).
Pain situation
After treatment, VAS and morphine dose in the observation group were both significantly decreased (P<0.05), however the morphine dose in the control group was not changed (P>0.05); the VAS in the control group was also decreased but not as much as the observation group (P<0.05) (Table 2).
| Stage | Observation group (n=81) | Control group (n=81) | ||
|---|---|---|---|---|
| VAS (score) | Morphine dose (mg) | VAS (score) | Morphine dose (mg) | |
| Before treatment | 7.47 ± 1.96 | 26.40 ± 2.97 | 7.39 ± 1.85 | 26.32 ± 3.19 |
| 15 d after treatment | 4.08 ± 1.26# | 20.30 ± 1.86 | 5.26 ± 1.37*# | 25.44 ± 3.30* |
| 15 d after treatment | 2.61 ± 1.33# | 15.51 ± 1.42 | 3.39 ± 1.65*# | 24.91 ± 3.52* |
| 21 d after treatment | 1.45 ± 0.38# | 10.68 ± 1.19 | 3.26 ± 1.30*# | 25.05 ± 3.37* |
Note: *P<0.05 compared with the observation group; #P<0.05 compared before treatment.
Table 2. Comparison of the VAS and morphine dose during treatment between two groups (x ± s).
Serum factors
After treatment, the serum VEGF and HGF were both significantly decreased in the observation group (P<0.05), but were not changed in the control group (P>0.05) (Table 3).
| Stage | Observation group (n=81) | Control group (n=81) | ||
|---|---|---|---|---|
| VEGF (pg/mL) | HGF (pg/mL) | VEGF (pg/mL) | HGF (pg/mL) | |
| Before treatment | 380.83 ± 90.51 | 399.43 ± 86.62 | 376.58 ± 90.39 | 397.24 ± 87.19 |
| 15 d after treatment | 279.60 ± 83.39# | 339.26 ± 85.54# | 385.62 ± 89.83* | 388.56 ± 84.43* |
| 15 d after treatment | 227.36 ± 70.91# | 284.62 ± 70.19# | 388.63 ± 87.42* | 391.36 ± 89.91* |
| 21 d after treatment | 267.36 ± 71.52# | 291.33 ± 60.25# | 385.40 ± 94.50* | 385.24 ± 87.40* |
Note: *P<0.05 compared with the observation group; #P<0.05 compared before treatment.
Safety
The vital signs in the two groups were not obviously changed, and there was no adverse reaction such as skin burn or organ perforation.
Follow up
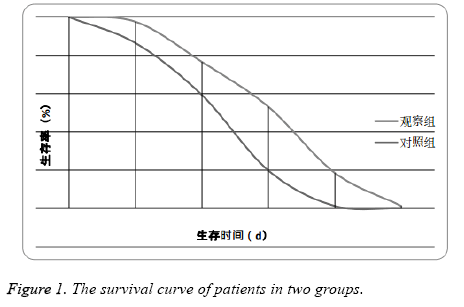
All the patients in the two groups were effectively followed up, the average follow up time was (23.2 ± 9.1) months. The median survival time (MST) in was (6.4 ± 1.3) months in the observation group and (3.6 ± 0.8) months in the control group. The MST in the observation group was significantly higher than the control group (P<0.05) (Figure 1).
Discussion
Pancreatic cancer is one of the malignant cancers that have the worst prognosis, and at present the MST of advanced pancreatic cancer is only 3-4 months [17,18]. To the patients who don’t meet the indications of surgery, the most common therapeutic regimen is radiochemotherapy or combined therapy. However, the tolerance of those patients is poor. Although the above treatment can inhibit the progress and metastasis of the cancer to a certain extent, it has no obvious effect on improving the surviving of patients [19-24]. The pain caused by pancreatic cancer is an important reason that decreases the life quality of patients [25-27]. Thus, some scholars suggest prolonging the surviving of patients by palliative treatment combined with analgesic drugs.
In this study, the potent opioid drug morphine was selected for analgesic treatment. After 21 days when the treatment was completed, VAS in the control group was significantly decreased, suggesting that morphine is effective in inhibiting cancerous pain. Morphine can simulate the effect of endogenous analgesic substance enkephalin to active opioid receptor in central nervous system, which can cause analgesic effect just like in a dream. This effect is optimal to the continuous dull pain caused by cancer. Meanwhile, morphine can decrease the excitability of central nervous system, and alleviate the tension and anxiety of patients to a certain extent, which is significant in improving the confidence of patients, ensuring the compliance and tolerance of patients [23,28]. However, some scholars point out that long term administration of morphine will cause drug addiction, decrease drug efficacy, also increases the smooth muscle tension and decrease sensitivity of respiratory center to carbon dioxide which easily causes respiratory failure and even death [23,24]. In this study, the morphine dose is not decreased during the treatment in the control group, suggesting that the alleviation of the pain is completely dependent on analgesic drug, which also verified the above conclusion.
The intermediate frequency ultrasound presented by high intensity focused ultrasound has received extensive attention. The technical principle is that due to the focusing and penetrativity of ultrasound wave in the body, it can produce instantaneous high temperature, and the heat effect and cavitation effect caused by local high energy can rapidly solidify the cancer cells to cause the degeneration and necrosis [29]. In this study, high intensity focused ultrasound was applied based on morphine analgesic in the observation group and we got better analgesic effect. The CBR was up to 84.0%. To further analyze the mechanism we also detected VEGF and HGF and found that VEGF and HGF were continuously decreased during the treatment. The growth of tumor is dependent on the formation of new vessels. VEGF can combine with the vesicae in the cytoplasm to form membrane channels, which provides the condition for the passing through of big molecule substance such as protein. This can increase the permeability of vessels, promote the formation and progress of cancer [30-33]. HGF is a polypeptide growth factor, which can promote the angiogenesis of cancer vessels, however the mechanism remains unclear [32,33]. In the control group, the VEGF and HGF levels were not obviously changed during the treatment, which might be the main reason that causes a poor MST. We consider that high intensity focused ultrasound can kill the local cancer cells, and also control the VEGF and HGF levels, which is the key to improve the life quality of patients. In this study, VEGF and HGF were increased slightly after the treatment was finished, which also verified that this was beneficial from intermediate frequency ultrasound.
The body of advanced stage pancreatic cancer patients is usually weak, and whether they can tolerate the treatment is the key of clinical efficacy. In this study, there was no obvious adverse effect in the observation group, suggesting that intermediate frequency ultrasound combined with opioid analgesic has good safety. However, we also found that the MST was only increased by 2 months, thus the efficacy still needs further improvement. This issue will be explored in the further study.
References
- Takasawa A, Murata M, Takasawa K, Ono Y, Osanai M. Nuclear localization of tricellulin promotes the oncogenic property of pancreatic cancer. Sci Rep 2016; 6: 33582.
- Luo J, Xiao L, Wu C, Zheng Y, Zhao N. The incidence and survival rate of population-based pancreatic cancer patients: Shanghai Cancer Registry 2004-2009. PLoS One 2013; 8: e76052.
- Butt Z, Parikh ND, Beaumont JL, Rosenbloom SK, Syrjala KL. Development and validation of a symptom index for advanced hepatobiliary and pancreatic cancers: the National Comprehensive Cancer Network Functional Assessment of Cancer Therapy (NCCN-FACT) Hepatobiliary-Pancreatic Symptom Index (NFHSI). Cancer 2012; 118: 5997-6004.
- Dumitra S, Jamal MH, Aboukhalil J, Doi SA, Chaudhury P, Hassanain M, Metrakos PP, Barkun JS. Pancreatic cancer and predictors of survival: comparing the CA 19-9/bilirubin ratio with the McGill Brisbane Symptom Score. HPB (Oxford) 2013; 15: 1002-1009.
- Panagiotarakou M, Gupta A, Syrigos K, Saif MW. Use of supportive care for symptom management in pancreatic cancer: application of clinical research to patient care. JOP 2012; 13: 342-344.
- Aroldi F, Bertocchi P, Savelli G, Rosso E, Zaniboni A. Pancreatic cancer: New hopes after first line treatment. World J Gastrointest Oncol 2016; 8: 682-687.
- Hirono S, Kawai M, Okada KI, Miyazawa M, Shimizu A. Treatment Strategy for Borderline Resectable Pancreatic Cancer With Radiographic Artery Involvement. Pancreas 2016; 45: 1438-1446.
- Karanikas M, Esempidis A, Chasan ZT, Deftereou T, Antonopoulou M. Pancreatic Cancer from Molecular Pathways to Treatment Opinion. J Cancer 2016; 7: 1328-1339.
- Jorand R, Biswas S, Wakefield DL, Tobin SJ, Golfetto O. Molecular signatures of mu opioid receptor and somatostatin receptor 2 in pancreatic cancer. Mol Biol Cell 2016; 27: 3659-3672.
- Skipworth RJ, Moses AG, Sangster K, Sturgeon CM, Voss AC, Fallon MT, Anderson RA, Ross JA, Fearon KC. Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support Care Cancer 2011; 19: 391-401.
- Zagon IS, McLaughlin PJ. Opioid growth factor and the treatment of human pancreatic cancer: a review. World J Gastroenterol 2014; 20: 2218-2223.
- Li T, Wang YN, Khokhlova TD, D'Andrea S, Starr F, Chen H, McCune JS, Risler LJ, Mashadi-Hossein A, Hwang JH. Pulsed High-Intensity Focused Ultrasound Enhances Delivery of Doxorubicin in a Preclinical Model of Pancreatic Cancer. Cancer Res 2015; 75: 3738-3746.
- Vidal-Jove J, Perich E, del Castillo MA. Ultrasound Guided High Intensity Focused Ultrasound for malignant tumors: The Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason Sonochem 2015; 27: 703-706.
- Wang G, Zhou D. Preoperative ultrasound ablation for borderline resectable pancreatic cancer: A report of 30 cases. Ultrason Sonochem 2015; 27: 694-702.
- Bernhard J, Dietrich D, Glimelius B, Bodoky G, Scheithauer W. Clinical benefit response in pancreatic cancer trials revisited. Oncol Res Treat 2014; 37: 42-48.
- Bernhard J, Dietrich D, Scheithauer W, Gerber D, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, Saletti P, Bauer J, Figer A, Pestalozzi BC, Kohne CH, Mingrone W, Stemmer SM, Tamas K, Kornek GV, Koeberle D, Herrmann R, Central European Cooperative Oncology Group. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial--SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol 2008; 26: 3695-3701.
- Coupland VH, Konfortion J, Jack RH, Allum W, Kocher HM, Riaz SP, Luchtenborg M, Moller H. Resection rate, hospital procedure volume and survival in pancreatic cancer patients in England: Population-based study, 2005-2009. Eur J Surg Oncol 2016; 42: 190-196.
- Varadhachary GR, Evans DB. Rational study endpoint(s) for preoperative trials in pancreatic cancer: pathologic response rate, margin negative resection, overall survival or 'all of the above'? Ann Surg Oncol 2013; 20: 3712-3714.
- Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev 2012; 38: 843-853.
- Shan YF, Fang YF, Wang XQ, Jin R, Zhang QY. Experimental studies on treatment of pancreatic cancer with double-regulated duplicative adenovirus AdTPHre-hEndo carrying human endostatin gene. Pancreatology 2013; 13: 393-400.
- Skoura E, Syrigos KN, Saif MW. Preclinical research in treatment of pancreatic cancer. JOP 2013; 14: 384-387.
- Wong KK, Qian Z, Le Y. The Role of Precision Medicine in Pancreatic Cancer: Challenges for Targeted Therapy, Immune Modulating Treatment, Early Detection, and Less Invasive Operations. Cancer Tansl Med 2016; 2: 41.
- Motaghinejad M, Bangash MY, Hosseini P, Karimian SM, Motaghinejad O. Attenuation of morphine withdrawal syndrome by various dosages of curcumin in comparison with clonidine in mouse: possible mechanism. Iran J Med Sci 2015; 40: 125-132.
- Navarro-Zaragoza J, Martinez-Laorden, E, Mora L, Hidalgo J, Milanes MV, Laorden ML. Cardiac adverse effects of naloxone-precipitated morphine withdrawal on right ventricle: role of corticotropin-releasing factor (CRF) 1 receptor. Toxicol Appl Pharmacol 2014; 275: 28-35.
- Dobosz ?, Stefaniak T, Dobrzycka M, Wieczorek J, Franczak P. Invasive treatment of pain associated with pancreatic cancer on different levels of WHO analgesic ladder. BMC Surg 2016; 16: 20.
- Erdek MA, King LM, Ellsworth SG. Pain management and palliative care in pancreatic cancer. Curr Probl Cancer 2013; 37: 266-272.
- Niu L, Wang Y, Yao F, Wei C, Chen Y, Zhang L, Chen J, Li J, Zuo J, Xu K. Alleviating visceral cancer pain in patients with pancreatic cancer using cryoablation and celiac plexus block. Cryobiology 2013; 66: 105-111.
- Andersson M, Bjorkhem-Bergman L, Beck O. Possible mechanism for inhibition of morphine formation from 6-acetylmorphine after intake of street heroin. Forensic Sci Int 2015; 252: 150-156.
- Gao HF, Wang K, Meng ZQ, Chen Z, Lin JH, Zhou ZH, Wang P, Shi WD, Sheng YH. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepato-gastroenterology 2013; 60: 1906-1910.
- Bhattacharya R, Ye XC, Wang R, Ling X, McManus M, Fan F, Boulbes D, Ellis LM. Intracrine VEGF Signaling Mediates the Activity of Prosurvival Pathways in Human Colorectal Cancer Cells. Cancer Res 2016; 76: 3014-3024.
- Taurone S, Galli F, Signore A, Agostinelli E, Dierckx RA. [Corrigendum] VEGF in nuclear medicine: Clinical application in cancer and future perspectives (Review). Int J Oncol 2016; 49: 1766.
- Tan XG, Yang ZL. Expression of Ezrin, HGF, C-met in pancreatic cancer and non-cancerous pancreatic tissues of rats. Hepatobiliary Pancreat Dis Int 2010; 9: 639-644.
- Ziegler KM, Considine RV, True E, Swartz-Basile DA, Pitt HA, Zyromski NJ. Adipocytes enhance murine pancreatic cancer growth via a hepatocyte growth factor (HGF)-mediated mechanism. Int J Surg 2016; 28: 179-184.