Research Article - Biomedical Research (2016) Volume 27, Issue 4
Quantitative determination of active constituent in fructus trichosanthis and its anti-proliferative effect on cervical carcinoma HeLa cells
Cai-Hong Zhang1*, Qian-Qian Sun2, Jing-Jing Hu3
1Department of Obstetrics and Gynaecology, Changhai Hospital, Second Military Medical University, Shanghai 200433, PR China
2Department of Gynaecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai 200040, PR China
3Clinical Research Center, Changhai Hospital, Second Military Medical University, Shanghai 200433, PR China
- *Corresponding Author:
- Cai-Hong Zhang
Department of Obstetrics and Gynaecology
Second Military Medical University
China
Accepted date: March 21, 2016
Abstract
To establish a method for determination of quercetin content in fructus trichosanthis, and to observe the inhibitory effect of fructus trichosanthis extract on growth of cervical carcinoma HeLa cells. Active constituent was determined by HPLC with quercetin as the reference using 0.3% phosphoric acid: methanol solution (47:53) as the mobile phase at a detection wavelength of 360 nm. MTT assay was used to determine the cell metabolic rate, while flow cytometry was utilized to observe the changes in apoptosis. Quercetin showed good linearity within a 1.0468-10.468 mg/mL range (r=0.9997), with an average recovery of 98.58%. Compared to the control group, different concentrations of fructus trichosanthis extracts all inhibited HeLa cell proliferation after acting on for 24 and 48 h. Moreover, inhibition rates increased significantly with increasing drug concentrations and prolonging treatment time, showing a certain time-dose-response relationship. S phase ratio of HeLa cells rose from 24.68% in the control group to 42.15%, while G2 phase cell ratio dropped from 10.54% in the control group to 5.11%. Fructus trichosanthis extract could arrest HeLa cells in S phase. Compared to the control group, percentage of S phase cells increased, while G2 phase cells decreased. And such effect was dosedependent. The present quantitative determination method is reproducible, accurate and reliable. Fructus trichosanthis extract has a significant inhibitory effect on the growth of HeLa cells in vitro.
Keywords
Fructus trichosanthis, Quercetin, Content determination, MTT, Flow cytometry.
Introduction
Chinese drug fructus trichosanthis is the dried ripe fruit of cucurbitaceous plants Trichosanthes kirilowii Maxim or Trichosanthes rosthornii Harms. Commonly known as Gualou or Diaogua, fructus trichosanthis has heat- clearing, phlegmeliminating, chest-loosening, bind-dissipating, drynessmoistening and bowel-lubricating functions, which is used for treatment of lung heat cough, phlegm turbidity, chest obstruction, cardiac pain, abdominal fullness, acute mastitis, lung abscess, acute appendicitis, constipation, etc., and is a key treatment for chest obstruction [1]. In recent years, with the widespread application of Chinese drug extraction, isolation, purification and structural elucidation technologies, scholars at home and abroad have made extensive studies on the active constituents of fructus trichosanthis, who have analyzed nearly a hundred types of compounds, including lipids, sterols, flavonoids, triterpenes, amino acids, proteins, etc [2-6].
In addition to major pharmacological actions such as cardiovascular system improving, expectorant, antitussive and anti-tumor properties, fructus trichosanthis also has antibacterial, anti-ulcer and purgative effects. This study investigates the quality standards for fructus trichosanthis extract using quercetin as the reference and studies its antiproliferative effect on human cervical carcinoma HeLa cells [7-12].
Materials
Instruments and reagents
MTT (SIGMA); RPMI1640 medium (GIBCO); acridine orange (SIGMA, MKBJ4170V); automated microplate reader (BIOTEC, USA); CO-150 CO2 incubator (NBS, USA); clean bench (Antai, Suzhou Purification Group); flow cytometer (BD); 1260 HPLC system (Agilent); 1260 DAD (Agilent); HPLC grade reagent.
Drug and cell
Chinese drug fructus trichosanthis was purchased from a pharmacy, which was identified as the dried ripe fruit of cucurbitaceous plant Trichosanthes kirilowii Maxim. Human cervical carcinoma HeLa cells were purchased from China Medical University.
Method
Chromatographic conditions
RP-C18 column (250 mm × 4.6 mm, 5 um); detection wavelength: 360 nm; mobile phase: 0.3% phosphoric acid: methanol = 47: 53; column temperature: room temperature. Volume flow rate: 1 mL/min.
Linearity range
Reference solution: An appropriate amount of quercetin reference was accurately weighed and added with methanol to prepare a 10.468 mg/mL reference solution.
Sample solution: About 2.0 g of fructus trichosanthis was accurately weighed, placed into a 100 mL round-bottomed flask, added with 50 mL of methanol solution, weighed, then extracted under heating reflux in a water bath for 3 h. After replenishing the weight loss, the extract was evaporated to dryness to remove methanol. The residue was added with 40 mL of methanol-25% HCl (4:1) mixed solution, heated to reflux for 40 min, then allowed to cool, transferred into a 50 mL volumetric flask and diluted to the mark with methanol. During analysis, the sample solution was filtered through 0.45 μm organic microporous membrane.
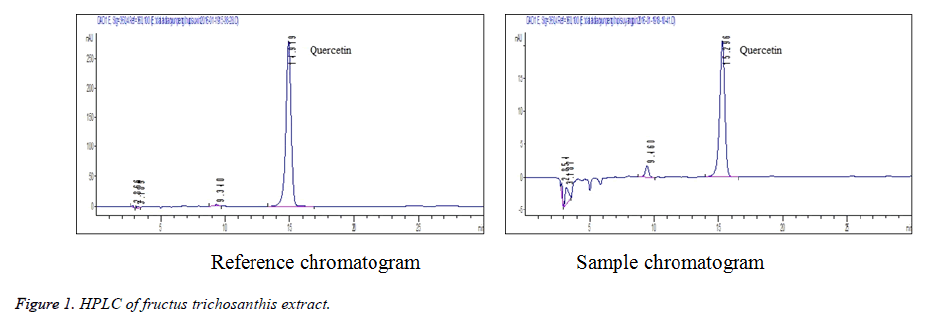
Linearity test: 0.5, 1, 2, 3, 4 and 5 mL of quercetin reference solution were accurately drawn, placed separately into 5 mL volumetric flasks, diluted to the mark with methanol and shaken well. The solutions were injected for determination under chromatographic conditions specified in section 2.1. Standard curve was plotted with peak area Y versus reference concentration X (ug·mL-1). Regression equation was obtained as follows: Y=577864X +4534 (r=0. 9997). The results showed that the quercetin was in good linearity within a 1.0468-10.468 mg/mL range (Figure 1).
Stability test: Each 20 μl of the same sample solution was accurately weighed and injected for determination at 0, 1, 2, 3, 4, 5 and 24 h, respectively. The results showed that RSD = 1.12%, indicating that the sample solution was stable within 24 h.
Accuracy test: Each 10 μL of reference solution was injected five consecutive times to measure the chromatographic peak areas, and RSD was found to be 2.3%.
Recovery test: Six aliquots of fructus trichosanthis with known contents were accurately weighed, added with corresponding amount of reference, prepared into sample solutions as per the method under section "2.2.2" and determined according to the above chromatographic conditions. Average recovery of six samples was 98.58%, RSD = 0.16%, indicating that the method yielded good recovery.
Reproducibility test: Five aliquots of samples from the same batch were accurately weighed and prepared as per the method for preparation of sample solution. Then, each 10 μl of the above solutions was accurately drawn and determined under the same chromatographic conditions. RSD was found to be 1.2% (n = 5).
Inhibition of HeLa cell proliferation by fructus trichosanthis extract
Cell culturing
HeLa cells were cultured in 10% FBS-containing DMEM medium and routinely subcultured in a 37, 5% CO2 incubator. Logarithmic phase cells were harvested for the experiment.
MTT assay of inhibitory effect on HeLa cells
Logarithmic phase HeLa cells were trypsinized for 2 min, then collected, density adjusted to 5 × 104 cells/mL with serum-free RPMI 1640 medium and seeded in 96-well plates at 100 μl cells/well. After culturing for 12 h, the medium was replaced with 10% FBS-containing RPMI 1640 medium. Wells were added with different concentrations of fructus trichosanthis extracts (20, 40, 80 mg/L), while control group was added with an equivalent volume of serum-free medium. Then culturing was continued for additional 24, 48 h. Five parallel wells were set up for each concentration. Before assay, each well was added with 20 μl of 5 mg/ml MTT solution and incubated for 4 h. Then supernatant was discarded, each well was added with 150 μl of DMSO solution, slightly shaken for 10 min and measured for absorbance at 570 nm with a microplate reader, followed by calculation of cell inhibition rate for each group.
Results
The results are shown in Table 1. Compared to the control group, different concentrations of fructus trichosanthis extracts could all inhibit the proliferation of HeLa cell after acting on for 24, 48 h. Moreover, the inhibition rates increased significantly with increasing drug concentrations and prolonging treatment time, showing a certain time-doseresponse relationship. Among them, high-dose group demonstrated inhibition rates on HeLa cell proliferation reaching 37.8% and 44.6%, respectively, showing statistically significant differences.
| Group | Concentration (mg/L) | Inhibition rate (%, 24 h) | Inhibition rate (%, 48 h) |
| Control group | 0 | 0 | |
| Fructus trichosanthis extract | 20 | 12.4 | 18.2 |
| 40 | 24.7 | 29.7 | |
| 80 | 37.8 | 44.6 |
Table 1. Inhibitory effects of fructus trichosanthis extracts on HeLa cells ( ± s)
HeLa cells treated by different concentrations of drugs and control group for 72 h were collected, respectively, and washed with pre-cooled PBS. Adherent cells were gently dissociated for suspension, placed in ethanol with a volume fraction of 0.80, fixed at 4 and treated with RNase (1 g.L-1) at 37 for 1 h. Samples were DNA fluorescent stained with PI, mixed well, incubated in the dark at room temperature for 15 min, then subjected to cell cycle analysis using a flow cytometer at 488 nm.
Effects of Fructus trichosanthis extracts on proportions of HeLa cells in various phases are shown in Table 2. The results showed that with increasing concentration of fructus trichosanthis extract, the proportion of S phase HeLa cells increased from 24.68% in the control group to 42.15%, while the proportion of G2 phase HeLa cells dropped from 10.54% in the control group to 5.11%. Fructus trichosanthis extracts could arrest HeLa cells in S phase. Compared to the control group, percentage of S phase cells increased, while G2 phase cells decreased. And such effect was dose-dependent.
| Group | Concentration (mg/L) | G0/ G1 phase | S phase | G2/ M phase |
| Control group | 64.78 ± 0.55 | 24.68 ± 0.34 | 10.54 ± 0.41 | |
| Fructus trichosanthis extract | 20 | 56.17 ± 0.78 | 28.98 ± 1.02 | 8.17 ± 0.32 |
| 40 | 57.36 ± 1.03 | 35.66 ± 0.93 | 6.98 ± 0.54 | |
| 80 | 52.74 ± 0.74 | 42.15 ± 1.14 | 5.11 ± 0.37 |
Table 2. Effects of fructus trichosanthis extracts on HeLa cell cycle (n = 6, ± s).
Discussion
Fructus trichosanthis has a long history of use as a medicine, which was originally recorded in the Shen Nong's Herbal Classic as a medium-grade drug. Fructus trichosanthis has multiple physiological activities, which is widely used in clinical practice, particularly in the treatment of cardiovascular diseases and tumors owing to its prominent efficacy. Besides, the herb is also characterized by rich source, low cost and small side effects. Establishment and improvement of quality standards for active constituents of fructus trichosanthis and investigation of anti-tumor mechanisms of fructus trichosanthis extract are of important significance to the development of fructus trichosanthis preparations.
Preliminary experiments on the quality standards of fructus trichosanthis showed that quercetin and flavonoid contents varied greatly among fructus trichosanthis of different origins. Fructus trichosanthis from Jiangsu, Hebei and Anhui contained relatively high levels of quercetin and flavonoids. In the selection of extraction solvent and method, orthogonal test was designed to investigate the effects of extraction solvents (60%, 80%, 95% ethanol and methanol solutions), extraction methods (ultrasonic extraction, reflux extraction and cold soaking), extraction time (2, 3, 4 h) on the content of fructus trichosanthis extract. Results revealed that extraction efficiency was the highest when methanol was used as the solvent; reflux extraction was superior to the other two methods; while extraction time of 3 or 4 h made little difference to the content. Considering the actual mass production, 3 h was finalized as the extraction time.
In the optimization of mobile phase, methanol-3% aqueous phosphoric acid (50:50), methanol-4% aqueous phosphoric acid (50:50) and methanol-5% aqueous phosphoric acid (50:50) were compared. The results found that methanol-4% aqueous phosphoric acid (50:50) yielded the best peak shape, high resolution, with no tailing. Thus, methanol-4% aqueous phosphoric acid (50:50) was selected as the mobile phase.
Qin Lin [13] et al. further observed the effects of fructus trichosanthis decoction on HeLa cells and macrophages by in vitro experiment, who found that at the same dosage of administration, low concentration fructus trichosanthis not only could inhibit HeLa cells, but also could promote macrophage growth. Fructus trichosanthis decoction exhibited marked inhibitory effect on HeLa cells, which was enhanced with increasing drug concentration. On macrophages, fructus trichosanthis had promoting and damaging dual effects.
Conclusions
To further clarify the antitumor mechanisms of fructus trichosanthis, MTT assay and flow cytometry were employed in the present study for mechanism determination. Under experimental conditions, compared to the control group, different concentrations of fructus trichosanthis extracts all inhibited HeLa cell proliferation after acting on for 24 and 48 h. Moreover, inhibition rates increased significantly with increasing drug concentrations and prolonging treatment time, showing a certain time-dose-response relationship. Among them, high-dose group demonstrated inhibition rates reaching 37.8% and 44.6%, respectively, showing statistically significant differences.
Flow cytometry showed that the S phase ratio of HeLa cells rose from 24.68% in the control group to 42.15%, while G2 phase cell ratio dropped from 10.54% in the control group to 5.11%. Fructus trichosanthis extract can arrest HeLa cells in S phase. Compared to the control group, percentage of S phase cells increased, while G2 phase cells decreased. And such effect was dose-dependent.
Main biochemical feature during apoptosis is chromatin condensation to fragments [14,15]. In the present experiment, morphologic changes in apoptotic cells can be clearly seen. Cells were rounded, and late apoptotic cells even presented apoptotic bodies. These results preliminary indicate that the anti-tumor effect of fructus trichosanthis is associated with induction of apoptosis.
References
- Jin GG, Liu SX. Arrangement of literatures on Fructus Trichosanthis. Journal of Chinese Medicinal Materials 1992; 15: 42-44.
- Kimura Y, Akihisa T, Yasukawa K, Takido M, Tamura T. Structures of 5 hydroxylated sterols from the seeds of trichosanthes-kirilowii maxim. Chemical and pharmaceutical bulletin 1995; 43: 1813-1817.
- Akihisa T. 7-Oxodihydrokaroundiol [7-oxo-D: C-friedoolean-8-ene-3α, 29-diol], a novel triterpene from Trichosanthes kirilowii. Chem Pharm Bull 1992; 40: 1199-1202.
- Akihisa T, Kokke WCMC, Kimura Y, Tamura T. Isokarounidiol (D: C-Friedooleana-6,8-diene-3.alpha.,29-diol): The first naturally occurring triterpene with a .DELTA.6,8-conjugated diene system. Iodine-mediated dehydrogenation and isomerization of its diacetate. Journal of Organic Chemistry 1993; 58: 1959-1962.
- Akihisa T. 7-oxo-10-cucurbitadienol from the seeds of Trichosanthes kirilowii and its anti-inflammatory effect. Phytochemistry 1994; 36: 153-157.
- Kimura Y. Cyclokirilodiol and isocyclokirilodiol: two novel cycloartanes from the seeds of Trichosanthes kirilowii Maxim. Chem Pharm Bull 1997; 45: 415-417.
- Wu B, Cao H, Chen SW, Wang MW, Yao XS. Protective effect of the extract of Frutus Trichosanthis on hypoxia and myocardial ischemia and reperfusion injury. Journal of Shenyang Pharmaceutical University 2000; 17: 450-451.
- Huang YM, Deng JZ, Xiao JS. Pharmacodynamics of Modified Gualou Xiebai Decoction extract. Journal of Chinese Medicinal Materials 2004; 27: 667-669.
- Shi Z, Dan SD, Yuan T, Gui YP, Cao CH, Zhang JF. Mechanism of trichosanthin induced apoptosis of mouse prostatic cancer RM-1 cells in vitro. Journal of Chinese Medicinal Materials 2009; 32: 239-242.
- Wang DM, Dai SY, Lu LL, Gao CH, Li Y. Experimental studies on protective effect of Pericarpium Trichosanthis extract on atherosclerosis in rats. Journal of Beihua University (Natural Science) 2008; 9: 128-131.
- Dou CM, Li JC. Effect of extracts of trichosanthes root tubers on HepA-H cells and HeLa cells. World J Gastroenterol 2004; 10: 2091-2094.
- Shawa PC, Lee KM, Wonga KB. Recent advances in trichosanthin, a ribosome-inactivating protein with multiplepharmacological properties. Toxicon 2005; 45: 683-689.
- Qin L, Gao WL. Effects of Frutus Trichosanthis on cervical carcinoma cells and macrophages. Journal of Shandong University of Traditional Chinese Medicine 1995; 19: 414-417.
- Marais S, Mqoco T, Stander A, van Papendorp D, Joubert A. The in vitro effects of a sulphamoylated derivative of 2-methoxyestradiol on cell number, morphology and alpha-tubulin disruption in cervical adenocarcinoma (HeLa) cells. Biomedical Research-India 2012; 23: 357-362.
- Murata-Hori M, Komatsu S, Uji Y, Hosoya H, Murai N. Concentration of singly phosphorylated myosin II regulatory light chain along the cleavage furrow of dividing HeLa cells. Biomedical Research 1998; 19: 111-115.