Research Article - Biomedical Research (2017) Volume 28, Issue 12
Quantitative and qualitative determination of gallic acid in Hedyotis diffusa Willd. extract and its anti-lung cancer NCI-H460 cell activity
Pengfei Li, Jijia Li, Yu Liu, Yingwei Guo, Yanan Liu and Yi Ren*Department of Thoracic Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning Province, PR China
- *Corresponding Author:
- Yi Ren
Department of Thoracic Surgery
Cancer Hospital of China Medical University
Liaoning Cancer Hospital and Institute,PR China
Accepted on April 19, 2017
Abstract
To establish a method for qualification and quantification of gallic acid in Hedyotis diffusa Willd. and to investigate its proapoptotic mechanism on lung cancer cells. Gallic acid content was determined by HPLC under the following conditions: stationary phase: Diamonsil C18 column; mobile phase: 0.2% methanol acetonitrile-0.1% phosphoric acid: 0.1% triethylamine aqueous solution (3:97); detection wavelength: 216 nm; and column temperature: 40°C. Apoptosis was detected with a flow cytometer by observing the apoptotic cell morphology, and livin, survivin protein expressions were detected by Western blot. Injection amount of gallic acid was in a good linearity with the peak area at a range of 0.02-0.41 μg, r=0.9997. Average recovery was 99.55%, RSD=2.25%. Hedyotis diffusa Willd. extract had a dose-dependent proapoptotic effect on NCI-H460 cells and suppressed the expressions of surviving and livin proteins in NCI-H460 cells. Quantitative determination of Hedyotis diffusa Willd. of different origins proves that the method is sensitive, accurate, reliable and reproducible. Induction of apoptosis in NCI-H460 lung cancer cells is achieved primarily by inhibiting the NCI-H460 cells from expressing survivin and livin, which provides a theoretical basis for use of Hedyotis diffusa Willd. extract for lung cancer treatment.
Keywords
Hedyotis diffusa Willd., Gallic acid, HPLC, NCI-H460 cell.
Introduction
Hedyotis diffusa Willd. (HHD) is bitter, flat and cold, which has antipyretic, detoxifying, analgesic, lump-resolving, diuretic and dehumidifying functions. It is especially effective for various types of inflammations and can be used for lung-heat dyspnea with cough, swollen sore throat, intestinal carbuncle, furuncles, venomous snakebites, heat strangury, edema, dysentery, enteritis, damp-heat jaundice, as well as various cancers. Modern pharmacological studies have shown that HHD has anti-inflammatory [1-3], immunoenhancing, antitumor, spermatogenesis suppressing and anti-snake venom effects [4-9]. Clinically, HHD is used for treating pediatric pneumonia, appendicitis, post-vasectomy epididymal stasis, pelvic inflammation, annexitis, benign thyroid nodules, etc.
At present, lung cancer has become the leading cause of cancer death in China. Preliminary experiments found that HHD extract had an inhibitory effect on NCI-H460 lung cancer cells, which was consistent with the literature reports. Nevertheless, what was studied was the extract of HHD, whose effective constituents were unclear. This paper aims to investigate the proapoptotic mechanism of HHD extract on lung cancer cells, in order to provide a theoretical basis for the treatment of lung cancer. Besides, the paper also aims to determine gallic acid,the major constituent of HHD, by HPLC; to control the quality of the extract; and to improve test reproducibility.
Instruments and Reagents
Agilent 1200 HPLC system; gallic acid reference (Chengdu Best Reagents Ltd.); HHD (Anguo Hengyu Medicines Ltd., batch number: 20151205A); HHD extract (self-prepared); NCI-H460 lung cancer cell line (Shanghai Suer Technology Co., Ltd.); HPLC grade acetonitrile; and all other reagents were of analytical grade. RPMI 1640, MTT and DMSO (Shanghai BioSun Sci and Tech Co., Ltd.). Rabbit anti-mouse IgG antibody; mouse anti-human β-actin, livin and survivin antibodies; and Western-blot Luminol (ECL) kit (Beijing Balb Biotechnology Co., Ltd.).
Chromatographic conditions
Column: octadecyl silane bonded silica filler (5 μm, 4.6 mm × 250 mm, Diamonsil C18); mobile phase: 0.2% methanol acetonitrile-0.1% phosphoric acid: 0.1% triethylamine aqueous solution (3:97); flow rate: 1.0 mL/min; detection wavelength: 216 nm; and column temperature: 40°C.
Methods
Selection of mobile phase and detection wavelength
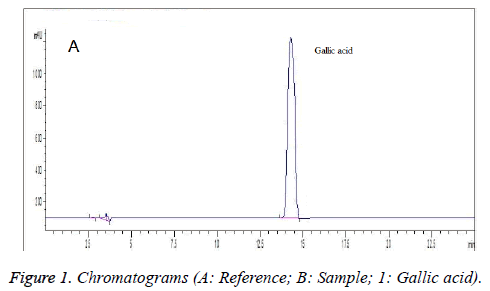
Comparison test of different mobile phases showed that under 0.2% methanol acetonitrile-0.1% phosphoric acid: 0.1% triethylamine aqueous solution (3:97), the peak of gallic acid in sample was baseline separated from the adjacent peaks, which was sharp and symmetrical in shape as well (Figure 1). Measurement of UV absorption spectrum of reference solution found that its maximum absorption wavelengths were 216 nm and 269 nm. Since gallic acid absorption was more sensitive at 216 nm, which could reduce the sampling amount, minimize the column contamination and allow steady baseline, 216 nm was selected as the detection wavelength. Figure 1 presents the chromatograms.
Preparation of reference solution
Appropriate amount of gallic acid reference was accurately weighed and added with 50% methanol to prepare an 8 μg/ml reference solution.
Preparation of sample solution and HHD extract
Appropriate amount of HHD was taken and crushed, 0.3 g of which was then accurately weighed and cold soaked in 30 ml of chloroform. After discarding the chloroform, the remaining was ultrasonicated with 50% methanol for 30 min, and then let cool and filtered. Afterwards, the filtrate was placed in a 50 ml volumetric flask, diluted to the mark with 50% methanol and shaken well to give the sample solution. According to the predetermined method, solvent was removed from the filtrate to yield an extract, which was precisely HHD extract.
Linear relation
0.0102 g of gallic acid reference was accurately weighed, placed in a 200 ml volumetric flask, diluted to the mark with 50% methanol and shaken well, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0 and 10.0 ml of which were then precisely drawn and placed separately in 50 ml volumetric flasks, diluted to the mark with 50% methanol and shaken well. Each 20 μL of reference solutions of different concentrations was precisely drawn and injected into the HPLC system for determination of gallic acid peak area. Standard curve was plotted with peak area as the ordinate and injection volume as the abscissa to obtain the regression equation: A=4963.95C-36.17, r=0.9997, which indicated good linearity within a gallic acid injection amount range of 0.02-0.41 μg.
Accuracy test
The same reference solution was precisely drawn and injected repeatedly five times for measurement of peak area. RSD was found to be 0.8%.
Reproducibility test
Samples of the same batch were taken and prepared into five sample solutions as per the above method for separate determination of gallic acid contents. The result revealed an average content of 1.47 mg/g, RSD=1.5%.
Stability test
Each 20 μL of sample solution was injected at 0, 2, 4, 8, 12 and 24 h, respectively, for measurement of peak area. The result revealed a RSD of 1.6%.
Recovery test
Six aliquots of 0.3 g of samples of the same batch containing 1.47 mg/g gallic acid were accurately weighed and added separately with 1.0, 1.0, 2.0, 2.0, 3.0 and 3.0 mL of 0.2152 mg/ml methanol solutions of gallic acid reference. After evaporating methanol to dryness, determination was performed according to the predetermined method, and recoveries were calculated, which were found to be 96.83%, 96.72%, 101.98%, 101.12%, 99.98% and 100.65%, respectively, with an average of 99.55%, RSD=2.25%.
Sample determination
Each 20 μL of reference and sample solutions were accurately drawn and determined according to the above chromatographic conditions. The results of gallic acid contents in samples are listed in Table 1.
| Batch | Content (mg/g) |
|---|---|
| 1 | 1.47 |
| 2 | 1.49 |
| 3 | 1.52 |
| 4 | 1.48 |
| 5 | 1.47 |
Table 1. Content determination results of HHD extract (n=3).
Cell culturing
NCI-H460 cells were seeded in RPMI1640 medium containing 10% FBS and cultured in a 37°C, 5% CO2 incubator. Logarithmic phase NCI-H460 cells were collected and added with different amounts of HHD extract to final concentrations of 0, 50, 100 and 200 mg/L, respectively, which corresponded to the control group, low-dose group, medium-dose group and high-dose group.
Morphological observation of apoptotic cells
Morphological changes in cells were observed by Hoechst 33342/PI immunofluorescence double staining. Hoechst 33342 was added to the cultured cells to a final concentration of 5 μg/ml and kept in the dark at 37°C for 10 min. Then, PI dye was added to the cells to a final concentration of 15 μg/ml and reacted in the dark at 4°C for 10 min, followed by observation with a fluorescence microscope.
FCM detection of apoptosis
1 × 105 cells were collected and washed twice with cold PBS. After resuspension, cells were stained with Hoechst 33342/PI, and apoptosis was detected by flow cytometry within 1 h. Fluorescence intensity of normal living cells was very low, green fluorescence intensity of apoptotic cells was high, while necrosis cells exhibited green and red double fluorescence. Apoptotic status of cells could be distinguished based on the fluorescence of cells.
Western blot detection of livin and survivin expressions
Four groups of cells, each containing 1 × 106 lysed cells, were taken for intracellular protein extraction to determine the protein concentration. Cellular proteins were transferred to the nitrocellulose membrane by SDS-PAGE gel electrophoresis and blocked at room temperature for 1 h. Afterwards, mouse anti-human actin, livin and survivin antibodies were added following the instructions, then horseradish peroxidase-labeled rabbit anti-mouse IgG antibody was added, followed by development by ECL reaction and exposure. Relative absorbance of bands (target protein absorbance/β-actin absorbance) was analysed using Quality One software, and protein expression levels were compared.
Statistical processing
Experimental data were expressed as x̄ ± s and statistically analysed using SPSS 17.0 software. Comparison between multiple sets of data was done by ANOVA at a significance level of α=0.05.
Results
Morphological changes of apoptotic cells
Under fluorescence microscope, the control group showed very low fluorescence intensity, while in the treatment groups, strongly green fluorescent apoptotic cells and red and green double fluorescent necrotic cells appeared. Apoptotic cells were rounded or shrunk, with intact membrane. Nuclear chromatin of partial cells condensed into blocks, lobules or unevenly thick fragments and separated from each other, which were unevenly dispersed in the cytoplasm. Vacuoles appeared in the cytoplasm to form apoptotic bodies. These suggested that HHD had a proapoptotic effect on NCI-H460 cells.
Apoptosis rate detection
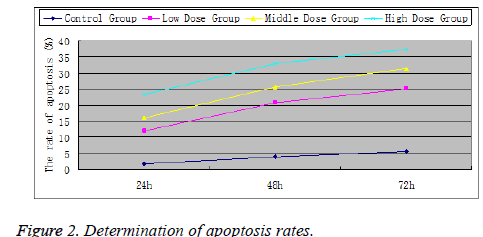
After treatment with HHD extract for 24, 48 and 72 h, the apoptosis rates in various treatment groups were all higher than the control group (P<0.05), indicating that the extract could effectively induce the apoptosis of NCI-H460 cells. Significant differences were found in the apoptosis rates among the low-, medium- and high-dose groups. Furthermore, with increasing extract concentration, apoptosis rate also increased accordingly, indicating the dose-dependence of HHD extract's proapoptotic effect on NCI-H460 cells (Figure 2).
Western blot detection of livin, survivin expressions
Experimental results found different expression levels of survivin and livin in all treatment groups. If the relative protein expression level was represented by the ratio of target protein absorbance to β-actin protein absorbance, survivin and livin expressions in various treatment groups would be all lower than the control group (P<0.01). As the HHD concentration increased, expressions of survivin and livin decreased gradually, with the lowest survivin and livin levels found in the high-dose group (Table 2). Experimental results demonstrated that HHD could inhibit the survivin and livin protein expressions in NCI-H460 cells, whose inhibition strength was associated with the concentration of HHD extract.
| Group | 0.39 ± 0.09** | 0.32 ± 0.09** |
| High-dose | 0.44 ± 0.12** | 0.46 ± 0.06** |
| Medium-dose | 0.71 ± 0.11* | 0.67 ± 0.12* |
| Low-dose | 0.97 ± 0.14 | 0.82 ± 0.10 |
| Control | 0.39 ± 0.09** | 0.32 ± 0.09** |
**Comparison with the control group, P<0.01; *comparison with the control group, P<0.05.
Table 2. Livin and survivin expressions in NCI-H460 cells.
Discussion
HHD contains a variety of chemical constituents, including liposoluble ones [10]. Low polar liposoluble constituents not only affect the separation of peaks, but also cause serious column contamination. Based on the chloroform insolubility of gallic acid, the present method firstly treats sample with chloroform, then extracts the sample with 50% methanol to yield satisfactory results.
The search for effective antineoplastics from the natural medicines is extremely important for lung cancer treatment. HHD has a marked antitumor activity, which can inhibit the proliferation of various tumor cells such as colon, lung and cervical cancer cells; suppress relevant signaling pathways such as nuclear transcription factor-KB (NF-KB), Mitogen- Activated Protein Kinase (MAPK) and epidermal growth factor receptor; and induce apoptosis of tumor cells [4,5,11]. Currently, there have been reports on the effect of HHD on lung cancer cells, which claim that HHD can induce A549 and SPCA-1 cell apoptosis. However, its proapoptotic mechanism on lung cancer cells is unclear yet, which requires further experimental study.
Livin and surviving are important Inhibitors of Apoptosis Proteins (IAP) discovered recently, which are specifically expressed only in the tumor and embryonic tissues [12-14]. Both contain domains inhibiting caspase proteases and play important roles in the occurrence and progression of tumors. Survivin is so far the strongest inhibitor of apoptosis, which is highly expressed in tumor cells such as esophageal, stomach, colon and breast cancer cells and can indirectly inhibit caspase activity by directly binding to caspase-9 or binding to auxiliary mitochondria-derived caspase activator to suppress apoptosis. Livin is a recently discovered novel IAP that is highly expressed in esophageal, stomach, breast and bladder cancer cells. After binding to caspase, Livin can block the protein removal function of caspase proteins, thereby inhibiting apoptosis.
This study found that after treatment of NCI-H460 cells with HHD extract, cells underwent apoptosis. Moreover, NCI-H460 cells intervened with the HHD extract expressed significantly decreased levels of livin and survivin proteins, indicating that the apoptosis-inducing effect of HHD extract was associated with livin and survivin downregulation.
Results of this study show that the HHD can induce apoptosis of NCI-H460 lung cancer cells and inhibit livin and surviving expressions in them, which provide a theoretical basis for the use of HHD extract for lung cancer treatment.
References
- Chen YL, Lin YY, Li YC, Li CD. Total flavonoids of Hedyotis diffusa Willd inhibit inflammatory responses in LPS-activated macrophages via suppression of the NF-κB and MAPK signaling pathways. Exp Ther Med 2016; 11: 1116-1122.
- Ye JH, Liu MH, Zhang XL, He JY. Chemical profiles and protective effect of Hedyotis diffusa Willd in lipopolysaccharide-induced renal inflammation mice. Int J Mol Sci 2015; 16: 27252-27269.
- Kuo YJ, Lin JP, Hsiao YT, Chou GL, Tsai YH, Chiang SY, Lin JG, Chung JG. Ethanol extract of hedyotis diffusa willd affects immune responses in normal Balb/c mice in vivo. In Vivo 2015; 4: 453-460.
- Lin J, Li Q, Chen H, Lin H, Lai Z, Peng J. Hedyotis diffusa Willd. extract suppresses proliferation and induces apoptosis via IL-6-inducible STAT3 pathway inactivation in human colorectal cancer cells. Oncol Lett 2015; 4: 1962-1970.
- Liu Z, Liu M, Liu M, Li JC. Methylanthraquinone from Hedyotis diffusa WILLD induces Ca2+-mediated apoptosis in human breast cancer cells. Toxicol In Vitro 2010; 24: 142-147.
- Kuo YJ, Yang JS, Lu CC, Chiang SY, Lin JG, Chung JG. Ethanol extract of Hedyotis diffusa willd upregulates G0/G1 phase arrest and induces apoptosis in human leukemia cells by modulating caspase cascade signaling and altering associated genes expression was assayed by cDNA microarray. Env Toxicol 2015; 30: 1162-1177.
- Zhang P, Zhang B, Gu J, Hao L, Hu F, Han C. The study of the effect of Hedyotis diffusa on the proliferation and the apoptosis of the cervical tumor in nude mouse model. Cell Biochem Biophys 2015; 3: 783-789.
- Niu Y, Meng QX. Chemical and preclinical studies on Hedyotis diffusa with anticancer potential. J Asian Nat Prod Res 2013; 5: 550-565.
- Lee HZ, Bau DT, Kuo CL, Tsai RY, Chen YC, Chang YH. Clarification of the phenotypic characteristics and anti-tumor activity of Hedyotis diffusa. Am J Chinese Med 2011; 39: 201-213.
- Wong KC, Tan GL. Composition of the essential oil of Hedyotis diffusa Willd. J Essential Oil Res 1995; 7: 537-539.
- Li W, Shen Z, Chen S, Lin P. Hedyotis diffusa Willd overcomes 5-fluorouracil resistance in human colorectal cancer HCT-8/5-FU cells by downregulating the expression of P-glycoprotein and ATP-binding casette subfamily G member 2. Exp Ther Med 2015; 10: 1845-1850.
- Zhang YM, Huang H, Zhou HM, Du T, Zeng LX, Cao Y, Chen JQ, Lai YM, Li J, Wang GP, Guo ZH. Activation of nuclear factor κB pathway and downstream targets survivin and livin by SHARPIN contributes to the progression and metastasis of prostate cancer. Cancer 2014; 20: 3208-3218.
- Kalungi S, Wabinga H, Bostad L. Expression of apoptosis associated proteins Survivin, Livin and Thrombospondin-1 in Burkitt lymphoma. Acta Pathologica Microbiologica Et Immunologica Scandinavica 2013; 121: 239-245.
- Wang JW, Zhang XD, Wei P, Zhang JH, Niu YN, Kang N, Zhang YX, Zhang WL, Xing NZ. Livin, Survivin and Caspase 3 as early recurrence markers in non-muscle-invasive bladder cancer. World J Urol 2014; 32: 1477-1484.