Research Article - Current Pediatric Research (2021) Volume 25, Issue 7
Putative effects of sexual hormones and cytokines in pre and post-menopausal women during bacterial urinary tract infection.
Ruaaabdal Kreemali1, Suha Maher Abed2, Firas Faris Rija2*
1Department of Biology, Salah Aldeen Health Directorate, Dijla Hospital for medical Rehabilitation, Tikrit, Iraq
2Department of Biology, College of Sciences, Tikrit University, Tikrit, Iraq
Corresponding Author:
- Firas Faris Rija
Department of Biology
College of Sciences
Tikrit University
Tikrit
Iraq
Email: firas_tucon@tu.edu.iq
Accepted date: July 27th, 2021
Abstract
Urinary Tract Infections (UTI) affect mostly females. The infection and possible consequent ascent of bacteria is enhanced by various risk factors. Sex hormones regulate gene transcription implicated in immune cell development and maturation, in regulation of immune responses and immune signaling pathways. This study was designed to determine the relationship between UTI and some sex hormones and role of them in cytokines related to immune response in pre and post-menopausal women during bacterial infection. The study included 90 urine samples from females age (18-69) years: forty five were pre-menopause group subdivided into two groups includes (25 infected with UTI and 20 control subjects) , while others forty five post- menopause group includes (25 infected with UTI and 20 control subjects) were attending to clinic of urology at Salahaldeen hospital and private medical clinic in Tikrit City. Blood samples were collected from these women to determine the concentrations of sex hormones (Estrogen (E2), Progesterone)and cytokines includes (Interleukin10 (IL-10),Transforming Growth Factor-β (TGF-β), Human β-Defensin 2 (HBD2) and Toll-Like Receptor4 (TLR-4) in those Sera, they assaying using ELISA technique assay . The positive isolates were selected for infected females of pre and post-menopausal. Fifty bacterial isolated were isolated from infected women with UTI, and the most frequently isolated microorganisms 27(54%) were Gram-negative bacteria; 15(60%) and 12(48%) pre and post-menopausal respectively, Followed by Gram-positive bacteria 23(46%) isolated; 10(40%) and 13(52%) pre and post-menopausal respectively. According to current research results of urine culture bacterial isolation, the most commonly isolated microorganisms were: Escherichia coli 15(30%) 8(32%), 7(28%) pre and post-menopausal respectively; and Enterobacter fecales in 10(20%) 5(20%), 5(20%) pre and post-menopausal respectively. The most effective antimicrobial agents were Amikacin, Gentamicin and Amoxicillin but the less effective were Ceftriaxone, Norfloxacin, and Nitrofurantoin. The results showed that the concentration of estrogen and progesterone hormones were higher in pre and post-menopausal without UTI (414.0 ± 34.2 pg/ml; 1.589 ± 0.460) pg/ml (406.1 ± 30.71 pg/ml; 1.894 ± 0.292) while decreased in pre and post-menopausal with UTI respectively; and decreased in the levels of IL-10, TGF-β, HBD2 and TLR-4 in a females with UTI pre and post-menopausal when compared without UTI females. The interaction between sex hormones and the incidence, progression and morbidity of UTI may be due to effective role of them at the levels of some cytokines like of IL-10, TGF-β, HBD2 and TLR-4. The ability of sex hormones to increase production of antimicrobial peptides, combined with the effect on epithelial integrity or distribution of proteins associated with cell-cell contact, to mention a few, place them as possible candidates for supportive treatment of UTI.
Keywords
Interleukin10 (IL-10), Transforming Growth Factor-β (TGF-β), Human β-Defensin2 (HBD2), Toll-Like Receptor-4 (TLR-4).
Introduction
Urinary Tract Infections (UTI) are one of the most prevalent and familiar types of bacterial infections responsible for a range of complications. UTI are among the most common healthcare-associated infections in catheterized patients, patients and residents in long-term facilities and also the most common infectious complication after kidney transplantation [1,2]. Uro Pathogenic E. Coli (UPEC) is generally responsible for over 80% of UTI, followed by S. saphrophyticus (5%- 15%). The remainingpercentage is mostly comprised of Klebsiella species and Proteus mirabilis [3]. The clinical picture of UTI ranges from asymptomatic and symptomatic bacteriuria, through acute and persistent UTI, to more complicated cystitis and pyelonephritis [4]. Generally, the risk factors include frequent sexual intercourse, contraceptive methods using spermicides, vaginal diaphragm, Depot Medroxy Progesterone Acetate (DMPA) or catheterization [2,5,6]. History of previous UTI or UTI in a first female relative, uncommon anatomical features or deformations, or pregnancy are considered risk factors for the development of UTI as well [5,6].
Clinical presentations between pre and post-menopausal women differ. Post-menopausal women are less likely to experience symptoms of lower UTI and more likely to suffer from ascending infection affecting kidneys [7]. Urinary incontinence, anatomical changes, menopause-associated oestrogen deficiency or other underlying morbidities, such as diabetes, are considered risk factors for elderly women [4]. Estrogens, mostly 17β-oestradiol (E2), have their primary role in reproductive tissues. E2 is secreted by ovaries and mainly promotes cell proliferation and growth of mammary glands and uterus [8].
Progesterone (P4) is produced in corpus luteum after ovulation and by placenta during pregnancy, as well as in adrenal glands and in the nervous system [9]. The immune system generally responds to UTI infection by innate immune defence, inflammatory mediators, cytokines and antimicrobial peptides. The barriersand tight junctions in the urothelium are also important during the immune response. Females generally present with greater number and activity of immune cells and inflammatory responses than males [10].
In addition, E2 can block the inflammatory effects caused by IL-1β and Lipo Poly Saccharide (LPS) from E. coli in human and rat uterus [6,4,9]. In bovine oviduct, LPS stimulation increases the secretion of IL-1β and TNF-α and it induces the expression of TLR2 and TLR4. However, E2 (1 ng/ml) reverses these inflammatory effects [11,12]. Human BetaDefensin-2 (HBD-2) is one of the most relevant Antimicrobial Peptides (AMPs) in the human reproductive tract, and its secretion is regulated by E2. For example, human uterine epithelium exposed to Lipo Poly Saccharide (LPS) in the presence of E2 (10m−3m) shows increased secretion of the AMPs, calcineurin-like metallophosphoesterase Super Family Protein (SLP1), and HBD-2, as well as decreased production of pro inflammatory cytokines via NF-κB inhibition [13].
Materials and Methods
Study design
Ninety individuals, serum and urine samples were collected; forty of them were normal without UTI subdivided into (20 subjects as pre and 20 were post-menopausal) and fifty cases were patients infected with UTI subdivided into(25 subjects as pre and 25 were post-menopausal) who attended to Clinic of Urology at Salahaldeen hospital and private medical clinic in Tikrit City. Patients and control groups females were between 18-69 years. All the control persons were non-diabetics and non-smokers with neither a familial history of diabetes nor a personal history of hypertensive, thyroid, or renal diseases.
Sample collection
Mid-Stream Urine (MSU) were collected in sterile container after telling the patients to clean the peripheral site, about 10- 20 ml of urine were collected and transported to the laboratory within 30 minutes; then used for culturing, microscopically, and biochemical examinations. Peripheral blood samples were also collected.
Culture conditions and identification
Bacterial identification was conducted depending onmicroscopic, culture characteristics, and biochemical tests under aerobic conditions which involved indole test to investigate production of indole, methyl red test to investigate sugar fermentation with acid production, vogus-proscauer test for acetone compound detection, utilization of citrate as a sole source of carbon and formation of sodium carbonate and urease test was done to indicates the hydrolysis of urea and the formation of ammonium, also oxidase test was used to investigate production of the Cyto-chrome, as well as performing catalase test and fermentation test for sugars, plus motility screening test and coagulase enzymes tests in two ways (slide and tube method) and lastly performing novo biocine susceptibility test to distinguish S. saprophyticus.
Antibiotics susceptibility test
Antibiotics susceptibility test was performed using Kirby-bauer method following standards of Clinical and Labo-Standards Institute (CLSI) Guidelines, the bacteria were classified as being (R) Resistant/Intermediate (I)/Susceptible (S)/for each antibiotic.
Hormonal and immunological assays including (E2): Progesterone and cytokines includes IL-10, TGF-β, HBD2 and TLR-4, estimated by using Enzyme Linked Immunsorbent Assay (ELISA) Sunlong Biotech Company kits with the sandwich method [14].
Statistical analysis: It was done by using SPSS, 2001 statistical program, and a comparison was made between various groups, which were evaluated by t-test. The level of statistical significance was calculated at (P˂0.05).
Results
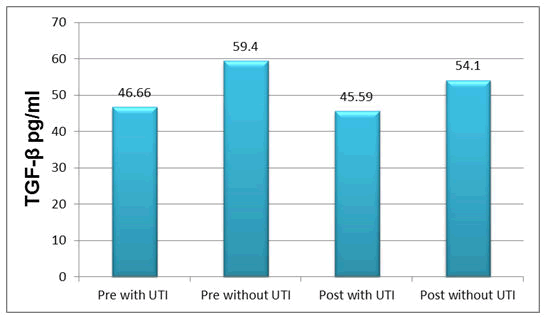
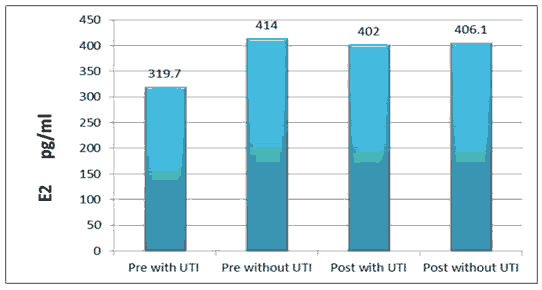
The results of the current study illustrated that the mean ± SD of sex hormones levels were (E2=319.7 ± 33.2 pg/ml, progesterone=1.529 ± 0.461 ng/ml) (E2=414.0 ± 34.2 pg/ml, progesterone=1.589 ± 0.460 ng/ml) pre with TUI and without UTI respectively with significant difference (P ≤ 0.05). Serum TGF-β was (46.66 ± 11.44), (59.4 ± 14.6) pg/ml in pre with TUI and without UTI respectively, while (45.59 ± 12.0), (54.1 ± 14.27) pg/ml in post with TUI and without UTI respectively.
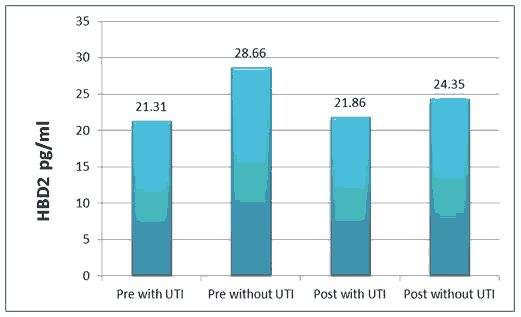
HBD2 level was (21.31 ± 2.65), (28.66 ± 3.80) pg/ml in pre with TUI and without UTI respectively, while (21.86 ± 2.82), (24.35 ± 3.48) pg/ml in post with TUI and without UTI respectively.
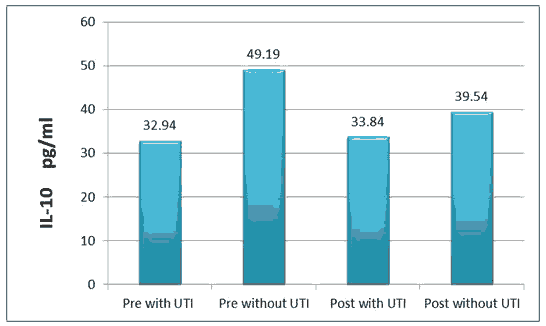
The result of IL-10 was (32.9 ± 43.42), (49.19 ± 3.64) pg/ml in pre with TUI and without UTI respectively, while (33.84 ± 6.67), (39.54 ± 5.79) pg/ml in post with TUI and without UTI respectively.
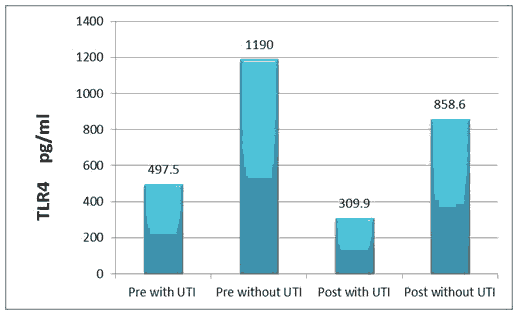
T L R-4 level was (497.5 ± 53.5), (1190.0 ± 39.9) pg/ml in pre with TUI and without UTI respectively, while (309.9 ± 46.4), (858.6 ± 27.7) pg/ml in post with TUI and without UTI respectively.
All the above results are shown in Tables 1 and 2, Figures 1-6.
| Groups | No. of subjects | Progestron | E 2 | T L R-4 | IL-10 | HBD2 | TGF-ß |
|---|---|---|---|---|---|---|---|
| Pre-UTI | 25 | 1.529 ± 0.461 | 319.7 ± 33.2 | 497.5 ± 53.5 | 3.42 ± 32.94 | 21.31 ± 2.65 | 46.66 ±11.44 |
| b | b | c | c | a | a | ||
| Post-UTI | 25 | 1.907 ± 0.341 | 402 ± 43.4 | 309.9 ± 46.4 | 33.84 ± 6.67 | 21.86 ± 2.82 | 45.59 ±12.0 |
| a | a | c | bc | a | a | ||
| Pre-Without | 20 | 1.589 ± 0.460 | 414 ± 34.2 | 1190 ± 39.9 | 49.19 ± 3.64 | 28.66 ± 3.80 | 59.4 ±14.6 |
| b | a | a | a | a | a | ||
| Post-Without | 20 | 1.894 ± 0.292 | 406.1 ± 30.71 | 858.6 ± 27.7 | 39.54 ± 5.79 | 24.35 ± 3.48 | 54.1 ±14.27 |
| a | a | b | b | a | a | ||
| P-Value | 0.001 | 0.0006 | 0.00009 | 0.042 | 0.307 | 0.729 |
Table 1. Serum levels of all study parameters in patients and control groups.
• Different letters indicate significant differences.
• Level of statistical significance at (P˂0.05).
| Isolated Bacteria | Pre-menopausal | Post-menopausal | Total |
|---|---|---|---|
| Escherichia coli | 8 | 7 | 15 (30%) |
| Enterobacter fecales | 5 | 5 | 10 (20%) |
| Streptococcus aureus | 3 | 6 | 9 (18%) |
| Klebsiella pneumonia | 4 | 3 | 7 (14%) |
| Pseudomonas aeruginosa | 3 | 2 | 5 (10%) |
| Staphylococcus saprophyticus | 2 | ||
| Total | 25 | 2 | 4 (8%) |
Table 2. Isolated bacteria from urine samples of pre and post-menopausal females.
Discussion
This study revealed that E2, progesterone, TLR4 and IL-10 levels decreased with a significant difference (P ≤ 0.05) in the pre and post with TUI patients group rather than control; while the HBD2 and TGF-β serum levels decreased but without significant difference (P ≤ 0.05).
UTI in postmenopausal women is higher prevalence in that period, rates of bacterial growth in UTI were in high prevalence especially Enterobacteriacea, abnormalities of immune system might result in a higher risk of certain physiological and bacterial infections, including UTI, immunologic impairments, such as impaired migration, intracellular killing, and phagocytosis.
Marital status and a history of recurrent UTI in premenopausal women have been associated with recurrent infections [15-18]. Another study described of lack of estrogen among diabetic and non-diabetic postmenopausal women appears to be an important factor predisposing to recurrent UTI [19,20]. Because the hormonal status plays an important role in the maintenance of a low vaginal pH, by estrogen induced increase in epithelial cell metabolism of glycogen, resulting in a lower vaginal pH, that's yield an environment less suitable to anaerobes. Post menopause is characterized by a significant reduction in ovary estrogen secretion, which is often associated with vaginal atrophy. Clinically, it associated by vaginal dryness, itching, and urinary incontinence, which cause a UTI. Estrogen induces the proliferation of lactobacillus in the vaginal epithelium, so reduces pH, and avoids vaginal colonization of Enterobacteriaceae, the main pathogens of the UTI.
The transition to menopause, characterized by declining estradiol levels, has an impact on immune senescence increasing the susceptibility to infection diseases and decreasing the efficacy of vaccination. These effects in postmenopausal women are characterized by an up regulation in the production of the cytokines TNF-α, IL-1β, IL-10, and IL-6, the stimulation of the cytotoxic activity of NK cells, and adiminished phagocytic capacity of Dendritic Cells (DCs), which leads to impaired antigen presentation and activationof the adaptive immune system [21].
Moreover, in vaginal epithelium, E2 (2 nM) induces HBD-2 secretion in response to LPS [22]. In addition, growth inhibition of S. aureus in the presence of E2 has been reported in a rat uterus and this inhibition is attributed to AMP secretion in the uterine lumen [23]. In the urinary tracts of women treated with E2 (10-3 M), a lesser recurrence of Escherichia coli infections has been observed, which is associated with the production of AMPs, such as defensins (HBD-1 and HBD-2), and thehomeostasis of the common microbial flora [13]. Several studies have demonstrated the regulation of the IIR of epithelia by E2. In all of the epithelial models where E2 plays an immune modulatory role, E2 has also been demonstrated to have anti-inflammatory effects. In human epithelial cells from the endometrium, E2 (10−7 M) shows an anti-inflammatory action reversing the effects caused by PAMPs, such as Poly I:C [24]. In addition, E2 can block the inflammatory effects caused by IL-1ß and Lipo Poly Saccharide (LPS) from Escherichia coli in human and rat uterus. In bovine oviduct, LPS stimulation increases the secretion of IL-1ß and TNF-a and it induces the expression of TLR2 and TLR4. However, E2 (1 ng/ml) reverses these inflammatory effects [11].
Conclusion
The innate immune system can function under hormonal control. Sexual hormones play an important role in the reproductive cycle of mammals, and it has immune modulatory effects on epithelial cells, which are the first line of defense against incoming bacteria. E2 regulates various pathophysiological processes, including the response to infection in epithelial cells, and its effects involve the regulation of innate immune signaling pathways, which are mediated through Estrogen Receptors (ERs). E2 modulates the expression of inflammatory and antimicrobial elements such as cytokines and antimicrobial peptides.
References
- Castañeda DA, León K, Martín R, et al. Urinary tract infection and kidney trarnsplantation: A review of diagnosis, causes, and current clinical approach. Transplant Poc 2013; 45(4):1590-2.
- Abbo LM, Hooton TM. Antimicrobial stewardship and urinary tract infections. Antibiotics (Basel) 2014; 3(2):174-92.
- Enderle JL, Miller AL, Pyles RB. Quantification of bacterial uropathogens in preclinical samples using realtime PCR assays. Curr Microbiol 2014; 68(2):220-6.
- Lüthje P, Hirschberg AL, Brauner A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas 2014; 77(1):32-6.
- Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 2012; 366(11):1028-37.
- Schmiemann G, Kniehl E, Gebhardt K, et al. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int 2010; 107(21):361-7.
- Foxman B. Urinary tract infection in postmenopausal women. Curr Infect Dis Rep 1999; 1(4):367-70.
- Wall EH, Hewitt SC, Case LK, et al. The role of genetics in estrogen responses: a critical piece of an intricate puzzle. FASEB J 2014; 28(12):5042-54.
- Schumacher M, Hussain R, Gago N, et al. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci 2012; 6(10):1-22.
- van Lunzen J, Altfeld M. Sex differences in infectious diseases - common but neglected. J Infect Dis 2014; 209(Suppl 3):S79-80.
- Kowsar R, Hambruch N, Liu J, et al. Regulation of innate immune function in bovine oviduct epithelial cells in culture: The homeostatic role of epithelial cells in balancing Th1/Th2 response. J Reprod Dev 2013; 59(5):470-8.
- Rija FF, Hussein SZ, Abdalla MA. Physiological and immunological disturbance in rheumatoid arthritis patients. Baghdad Sci J 2021; 18(2):247-52.
- Patel MV, Fahey JV, Rossoll RM, et al. Innate immunity in the vagina (part I): Estradiol inhibits HBD2 and elafin secretion by human vaginal epithelial cells. Am J Reprod Immunol 2013; 69(5):463-74.
- https://www.sunlongbiotech.com/category.php?id=66.
- Boyko EJ, Fihn SD, Scholes D, et al. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol 2005; 161(6):557-64.
- Stapleton A. Urinary tract infections in patients with diabetes. Am J Med 2002; 113(Suppl 1A):80S-4S.
- Hooton TM, Scholes D, Stapleton AE, et al. A prospective studies of asymptomatic bacteriuria in sexually active you women. N Engl J Med 2000; 343(14):992-7.
- Curran EM, Tassell AH-V, Judy BM, et al. Estrogen increase menopausal host susceptibility to experimental ascending urinary-tract infection. J Infect Dis 2007; 195(5):680-3.
- Brown JS, Vittinghoff E, Kanaya AM, et al. Urinary tract infections in postmenopausal women: Effect of hormone therapy and risk factors. Obstes Gynecol 2001; 98(6):1045-52.
- Reid G, Burton J. Use of lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect 2002; 4(3):319-24.
- Giefing-Kröll C, Berger P, Lepperdinger G, et al. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015; 14(3):309-21.
- Han JH, Kim MS, Lee MY, et al. Modulation of human β-defensin-2 expression by 17β-estradiol and progesterone in vaginal epithelial cells. Cytokine 2010; 49(2):209-14.
- Fahey JV, Rossoll RM, Wira CR. Sex hormone regulation of anti-bacterial activity in rat uterine secretions and apical release of anti-bacterial factor(s) by uterine epithelial cells in culture. J Steroid Biochem Mol Biol 2005; 93(1):59-66.
- Crane-Godreau MA, Wira CR. Effects of estradiol on lipopolysaccharide and Pam3Cys stimulation of CCL20/macrophage inflammatory protein 3 alpha and tumor necrosis factor alpha production by uterine epithelial cells in culture. Infect Immun 2005; 73(7):4231-7.