Research Article - The International Tinnitus Journal (2023) Volume 27, Issue 1
Pulsed Radiofrequency of the Auricular Branch of the Vagal Nerve in Tinnitus Patients
1Department of Pain therapy, Pain Clinic De Bilt, De Bilt, Netherlands
2Department of Neuroscience, Research Centre Neurosensory Science, School of Medicine and Health Science, Carl von Ossietzky University Oldenburg, Oldenburg, Germany
Send correspondence to:
Henk M. Koning
Department of Pain Therapy, Pain Clinic De Bilt, De Bilt, Netherlands, E-mail: hmkoning@pijnkliniekdebilt.nl
Tel: 302040753
Paper submitted on April 19, 2023; and Accepted on May 08, 2023
Citation:. Koning HM, Heeringa AN. Pulsed Radiofrequency of the Auricular Branch of the Vagal Nerve in Tinnitus Patients. Int Tinnitus J. 2023;27(1):68-74.
Abstract
Introduction: Pulsed radiofrequency of the auricular branch of the vagal nerve has strongly reduced tinnitus in a person with violent tinnitus and severe cervical pain.
Objectives: The objective of our study was to study the long-term effects of pulsed radiofrequency of the auricular branch of the vagal nerve in a large group of tinnitus sufferers and to find predictors for a prosperous result.
Design: A monocenter backward-looking group study.
Results: 48% of tinnitus sufferers who undertook pulsed radiofrequency of the auricular branch of the vagal nerve reported a reduced loudness of their tinnitus, which was qualified as being moderate to good in 87% of these patients. The reduction exceeded mostly 1 year. An angle smaller than 3 degrees between the 2nd and 3rd cervical vertebrae on lateral radiograph predicted a better outcome of this therapy.
Conclusion: Neuromodulation of the auricular branch of the vagal nerve is an uncomplicated remedy for tinnitus, especially for tinnitus patients with a pathologically small C2-C3 angle.
Introduction
Tinnitus is mostly initiated by damage to the peripheral auditory system, specifically by exposure to loud noise or a blast [1,2]. However, also (sensory) neural systems other than the auditory system can initiate and modulate tinnitus. Large-cohort tinnitus studies show that in 12% of patients who seek medical help for their tinnitus, the tinnitus started after head or neck trauma, such as a concussion or a whiplash [3]. Furthermore, 80% of people with non-clinical tinnitus (who did not seek medical help for their tinnitus) can modulate their tinnitus percept using head or neck movements and 60% of people without tinnitus can elicit a tinnitus percept upon head or neck movements.
These non-auditory neural systems involved in tinnitus also provide opportunities for the alleviation of tinnitus and the stress-related burden it causes. In laboratory animals, tinnitus, as determined in a behavioral paradigm, can be eliminated by invasive Vagal Nerve Stimulation (VNS) or transcutaneous stimulation of the facial nerve and dorsal root ganglion [4, 5]. However, the translation of such treatments to humans shows less-promising outcomes, where tinnitus burden is mitigated only temporarily and/ or in only a subpopulation of patients [5, 6]. The discrepancy between animal and human research could be that animal studies involve a homogeneous group of tinnitus animals with likely similar underlying pathological mechanism(s) and similar time of treatment after onset, whereas tinnitus in humans is manifested as highly heterogeneous [7]. To further disentangle tinnitus heterogeneity, we here study underlying causes of variability in treatment success following Pulsed Radiofrequency (PRF) of the Auricular Branch of the Vagal Nerve (ABVN).
The ABVN is a fiber bundle containing sensory fibers only, those branches from the vagal nerve and runs through the ear canal towards the brainstem. Stimulation can be using minimally invasive methods, by placing an electrode percutaneously at the inner tragus. Apart from the major vagal nerve branch, spinal, trigeminal, and facial nerves run close to the ABVN innervation area [8]. In the present study, the ABVN was targeted using PRF, which can alter the sensory nociceptors [9] and the electric stimulation switch on the Nucleus of the Solitary Tract (NTS) [10]. A previous and promising case study in a patient with lifethreatening tinnitus and cervical pain showed that PRF of the ABVN strongly reduced both tinnitus loudness and cervical pain [11]. Therefore, the intention of this inquiry is to check this treatment on a large group of tinnitus patients, estimate the long-term effect of PRF of the ABVN, and find clinical predictors for a prosperous result.
Methods
Design: A monocenter backward-looking group study in Pain Clinic De Bilt, De Bilt, the Netherlands.
Ethical Approval: The Ethics Committee United (Nieuwegein, the Netherlands) permitted this study (W22.103, May 7, 2022). Each patient gave informed permission to the procedure.
Subjects: This study includes all tinnitus patients who underwent PRF of the ABVN in Pain Clinic De Bilt between April 2020 and May 2022 (n=112). Other therapy options offered were counselling by a neurologist or ENTspecialist, medication advice, and therapy of ganglion C2 or ganglion cervicale superior; outcomes of these therapies are not analyzed in the current study. No further rejection criteria for PRF of the ABVN were used. Before therapy, each patient filled in a clinical questionnaire and qualified the loudness of their tinnitus on a visual analogue scale (0-100 mm). Furthermore, a two-sided audiogram and a radiograph of the neck were acquired before the treatment started.
Outcome: The prime outcomes were subjective change in tinnitus loudness at 7 weeks post treatment, measured as percentage of preoperative loudness, and time of sustained tinnitus relief following therapy.
Adverse Effects: Side effects were registered directly after and at 7 weeks post treatment.
Assessment of Hearing: A two-sided audiogram for the tone thresholds from 250 to 8000 Hz (in dB HL).
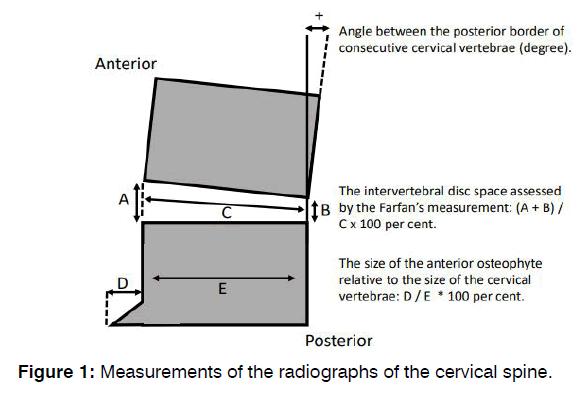
Radiograph of the Neck: Measurements of the radiographs of the cervical spine are described in Figure 1.
Pulsed Radiofrequency of the Auricular Branch of the Vagal Nerve: The treatment was executed by an anesthesiologist and carried out without sedation. After decontamination, a 22-gauge, 60 mm-long needle with a 5 mm active tip was positioned percutaneously at the inner tragus. Then pulsed radiofrequency at 42 V, 2 Hz, and 10 milliseconds for 10 minutes was applied. Postoperative, patients were monitored for 30 minutes. Patients were re-evaluated 7 weeks post treatment. Treatment was conducted to the ear where the tinnitus was the loudest.
Data Assessment: Obtained information included tinnitus features from the clinical questionnaire (dominant side of tinnitus, period of tinnitus, presence of diminished hearing, dizziness or balance disorders), self-reported profit at seven weeks post treatment on a four-point scale (none [0%], slight [<25%], moderate [25%-50%], good [50% or greater]), and duration of improvement. Seven weeks postoperative, additional therapy was offered. When more than one therapy was received, the improvement until the last therapy was documented and added in the survival analysis as period of ongoing tinnitus relief. All persons with a beneficial PRF of the ABVN and no recurrence nor other therapy, were invited for a question-and-answer session to value duration of improvement. If there was still tinnitus relief, it was added in the survival analysis as period of ongoing tinnitus relief. In August 2022, a survey with a standardized set of questions by an independent observer was accomplished.
Statistical Methods: Data were explored with Minitab 18 (Minitab Inc., State College, PA, USA) using Student’s t-test, χ2 test, and survival analysis. Discriminant analysis gets the measure how variables were bind to the outturn of PRF of the ABVN. A P-value smaller than 0.05 stipulated statistically meaningful.
Results
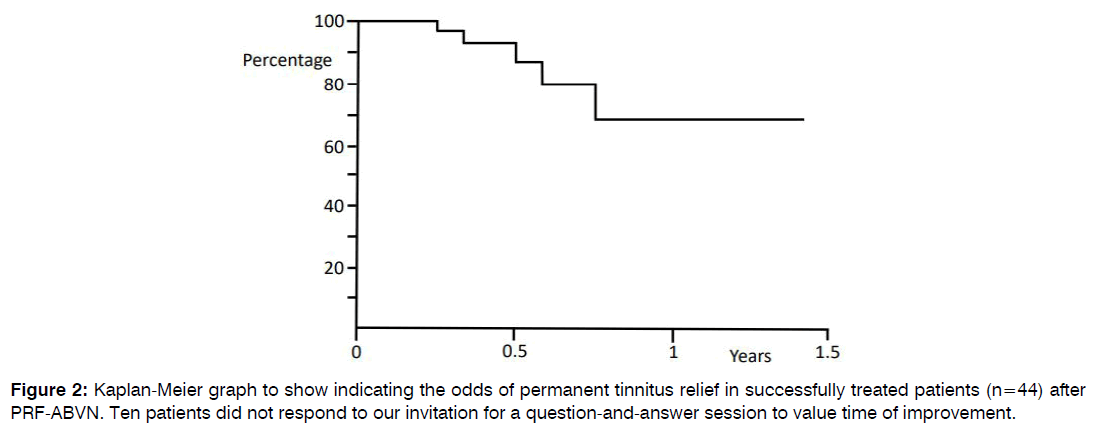
In a two-year period, 112 tinnitus patients had PRF of the ABVN. Table 1 displays the clinical parameters of the patients. Fifty-four patients (48%) reported reduced tinnitus loudness at the 7-week post-treatment follow-up. These patients valued the improvements as: 33% good, 54% moderate, and 13% slight. In 4% of patients, tinnitus magnified after the PRF of the ABVN treatment and one patient reported postoperative occurrence of dizziness. Patients were observed for up to 1.5 years postoperative. The odds of permanent tinnitus relief after successful PRF of the ABVN are 66% at one year postoperative (Figure 2).
| Prevalence | Median | Q1 – Q3 | |
|---|---|---|---|
| Age (year) | 57 | 50 – 66 | |
| Gender (male) | 58% | ||
| Unilateral tinnitus | 37% | ||
| Self-perceived hearing loss | 66% | ||
| Cervical pain | 72% | ||
| Period of tinnitus (year) | 5 | 2 – 15 | |
| Perceived intensity of tinnitus (mm) | |||
| Mean | 73 | 50 – 84 | |
| Minimal | 33 | 23 – 57 | |
| Maximal | 87 | 75 – 95 | |
| Hearing loss (dB) at: | |||
| 250 Hz | 15 | 7 – 35 | |
| 500 Hz | 15 | 9 – 34 | |
| 1 kHz | 15 | 10 – 40 | |
| 2 kHz | 22 | 10 – 45 | |
| 4 kHz | 40 | 23 – 60 | |
| 8 kHz | 55 | 30 – 70 |
Table 1: Clinical Characteristics of the patients with tinnitus.
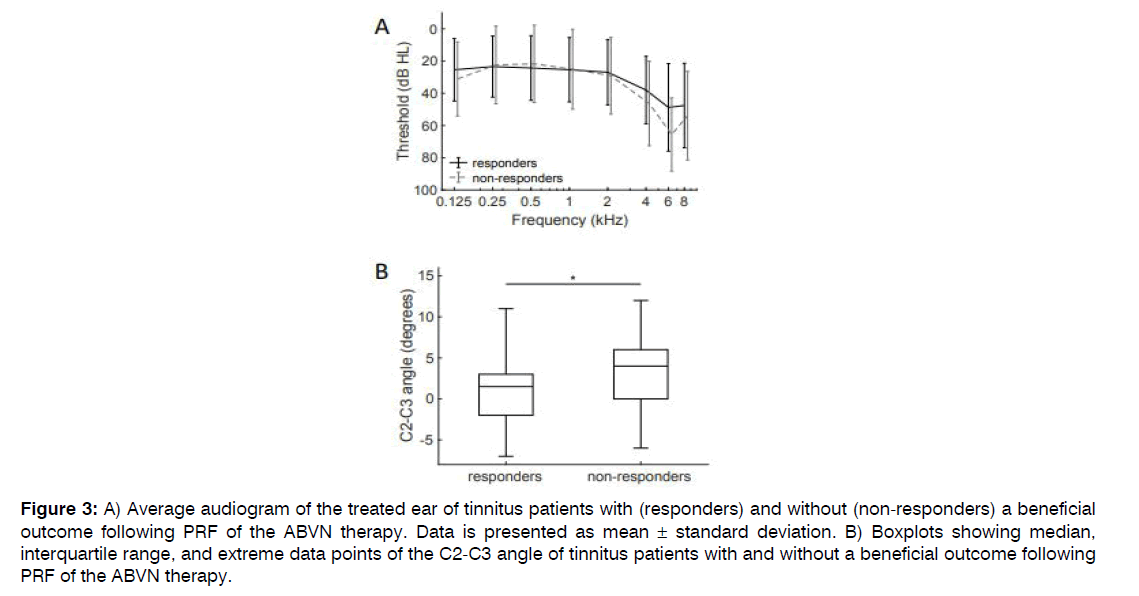
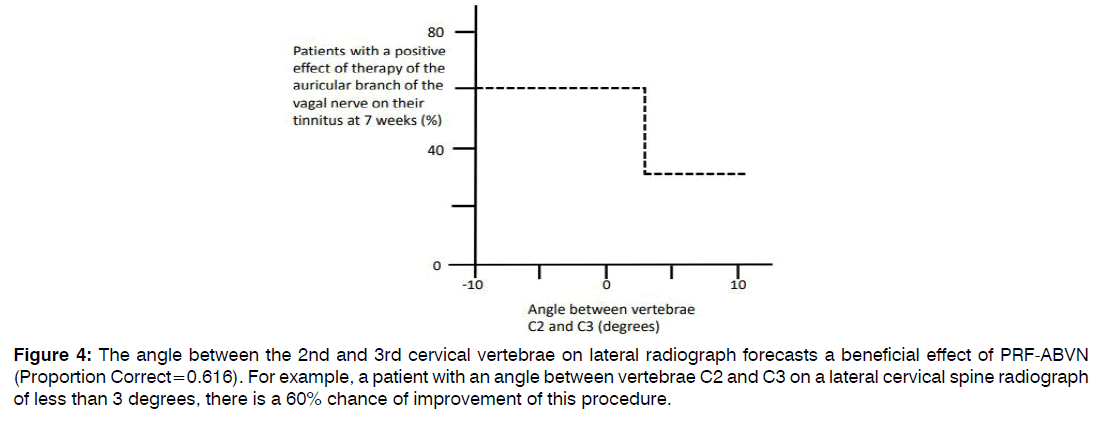
Patients with a beneficial result of PRF of the ABVN on their tinnitus were compared to patients that had no effect after PRF of the ABVN treatment (Table 2). Hearing thresholds at the treatment side were not dissimilar between patients with and patients without a beneficial effect after the ABVN stimulation therapy (responders and non-responders, respectively; Figure 3A). Of all clinical outcome measures, only the angle between the 2nd and 3rd cervical vertebrae was statistically significant distinct between responders and non-responders to the PRF of the ABVN therapy (p=0.014). A significantly reduced angle was noticed in tinnitus sufferers with a beneficial outcome of PRF of the ABVN (Figure 3B). With discriminant analysis, persons with more chance on a good outcome of PRF of the ABVN were distinguished (Figure 4). In tinnitus sufferers with an angle smaller than 3 degrees between the 2nd and 3rd cervical vertebrae on lateral radiograph, 60% had a beneficial effect of PRF of the ABVN compared to 36% in those not fulfilling these criteria. This criterion had a sensitivity of 60% and a specificity of 64% for predicting a beneficial response following PRF of the ABVN in tinnitus sufferers.
| Positive effect of therapy (n=54) | No effect of therapy (n=58) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Prev. | Mean | SEM | Prev. | Mean | SEM | |||
| Age (year) | 56 | 1.6 | 57 | 1.7 | 0.71 | |||
| Gender (male) | 54% | 62% | 0.37 | |||||
| Unilateral tinnitus | 37% | 36% | 0.927 | |||||
| Self-perceived hearing loss | 67% | 66% | 0.898 | |||||
| Cervical pain | 76% | 68% | 0.378 | |||||
| Age at the start of tinnitus (year) | 47 | 2 | 46 | 2.1 | 0.797 | |||
| Hearing loss (dB) at: | ||||||||
| 250 Hz | 23 | 2.7 | 22 | 3.2 | 0.807 | |||
| 500 Hz | 24 | 2.8 | 22 | 3.2 | 0.548 | |||
| 1 KHz | 25 | 2.8 | 25 | 3.3 | 0.939 | |||
| 2 KHz | 27 | 2.8 | 29 | 3.2 | 0.609 | |||
| 4 KHz | 38 | 2.9 | 46 | 3.5 | 0.071 | |||
| 8 KHz | 48 | 3.7 | 54 | 3.7 | 0.226 | |||
| Angle between vertebrae C2 and C6 (degrees): | 3 | 1.4 | 6 | 1.3 | 0.066 | |||
| Angle between vertebrae C2 and C3 (degrees): | 1 | 0.6 | 3 | 0.6 | 0.014 | Sign. | ||
| Farfan’s measurement of disc space height (%): | ||||||||
| C2-C3 | 40 | 1.2 | 39 | 0.9 | 0.868 | |||
| C3-C4 | 35 | 1.2 | 34 | 1.4 | 0.51 | |||
| C4-C5 | 34 | 1.3 | 32 | 1.2 | 0.345 | |||
| C5-C6 | 27 | 1.3 | 27 | 1.1 | 0.932 | |||
| C6-C7 | 27 | 1.3 | 26 | 1.4 | 0.617 | |||
| Size of anterior osteophyte (%) at: | ||||||||
| C3 | 8 | 0.7 | 10 | 1 | 0.108 | |||
| C4 | 12 | 1 | 13 | 0.9 | 0.492 | |||
| C5 | 19 | 0.9 | 19 | 1.2 | 0.831 | |||
| C6 | 14 | 0.9 | 16 | 1 | 0.342 | |||
Table 2: Patients with a positive effect of therapy of the auricular branch of the vagal nerve on their tinnitus at 7 weeks were compared with non-responders.
Figure 3: A) Average audiogram of the treated ear of tinnitus patients with (responders) and without (non-responders) a beneficial outcome following PRF of the ABVN therapy. Data is presented as mean ± standard deviation. B) Boxplots showing median, interquartile range, and extreme data points of the C2-C3 angle of tinnitus patients with and without a beneficial outcome following PRF of the ABVN therapy.
Figure 4: The angle between the 2nd and 3rd cervical vertebrae on lateral radiograph forecasts a beneficial effect of PRF-ABVN (Proportion Correct=0.616). For example, a patient with an angle between vertebrae C2 and C3 on a lateral cervical spine radiograph of less than 3 degrees, there is a 60% chance of improvement of this procedure.
Discussion
Involvement of the Cervical Spine and Spinal Nerves in Tinnitus: This study unveiled that a subset of tinnitus patients profited from PRF of the ABVN, who differed from other tinnitus patients only in terms of the angle between the 2nd and 3rd cervical vertebrae. On average, this angle was less than 3 degrees in patients who responded beneficial to the PRF-ABVN treatment. As the vagal nerve runs in front of the upper cervical vertebrae, an angle smaller than average (i.e., two degree) or below zero can irritate the vagal nerve and the spinal nerves. Specifically, the nerve can be irritated by the excessive motions of these vertebrae caused by cervical instability or by a kyphotic posture of the second cervical vertebrae. Hence, we can speculate that in patients who benefit from PRF of the ABVN, the tinnitus may be originated, maintained, or influenced by pathological activity of the spinal (sympathetic) and/or vagal nerves that run within the C2-C3 area. As fibers in the ABVN zone are all afferent, PRF of the ABVN likely alters the cervical spine-to-central nervous system pathway.
Alleviating tinnitus through stimulation of the cervical nerves has been shown in earlier studies in guinea pigs. Transcutaneous activation of the dorsal column reduced tinnitus-like behavior in all animals when paired with acoustic stimulation matching the tinnitus frequency, while reducing tinnitus behavior in half of the animals when presented without any acoustic stimulation [5]. These changes in tinnitus behavior strongly correlate to spontaneous activity and neural synchrony in the primary cells of the Dorsal Cochlear Nucleus (DCN) [12]. The DCN is an auditory nucleus in the brainstem, receiving its major input from the auditory nerve. However, the spinal trigeminal nucleus (Sp5) and cuneate nucleus also project to the cochlear nucleus and can modify activity in the DCN [13, 14]. Importantly, this Sp5 and cuneate nucleus to DCN innervation and auditory-somatosensory integration is changed in animals with tinnitus [15, 16]. Since the ABVN, or its hitchhiking nerves, also project to the Sp5 and the cuneate nucleus [17], this may be a mechanism by which PRF of the ABVN treatment alleviates tinnitus.
Involvement of the Vagal Nerve in Tinnitus: Since the ABVN contains fibers from the vagal nerve, the way by which PRF of the ABVN alleviates tinnitus may be similar to the way of tinnitus alleviation by direct VNS. For this, the following mechanisms have been suggested. Direct VNS, paired with sound, reverses maladaptive cortical tonotopic map reorganization [4]. Vagal nerve stimulation activates the NST, which subsequently activates the noradrenergic Locus Coeruleus (LC) and the cholinergic nucleus basalis of Meynert [18, 19]. The LC is the predominant source of noradrenaline modulation in the cerebrum, and projects to large portions of subcortical and cortical areas [20]. The nucleus basalis of Meynert forwards cholinergic input to the cerebral cortex and the amygdala [21, 22]. The LC and the amygdala actively determine which sensory signals are picked out for processing in sensory brain regions [23]. As such, tapping into the cholinergic system through VNS may reverse cortical, maladaptive plasticity related to tinnitus. Another way by which VNS is proposed to alleviate tinnitus, is through general parasympathetic activation, which reduces anxiety and tinnitus-related distress [5].
Previous studies demonstrated that when VNS was paired with sounds, it resulted in clinically meaningful improvements in 50% of tinnitus sufferers [19]. In our study, 48% of the patients experienced less tinnitus loudness and 4% of the patients had an aggravation of tinnitus after PRF of the ABVN. We obtained equal results without offering simultaneous sounds to patients while stimulating. This raises doubt whether offering sounds during VNS is beneficial and necessary. Indeed, when tinnitus loudness was evaluated in epilepsy patients that received VNS (without simultaneous acoustic stimulation), 80% showed less tinnitus loudness but arguably also stronger VNS stimulation [24, 25]. Possibly, the inclusion and recruitment of tinnitus patients may be pivotal for the outcome and for the selection of therapy with the most chance of success. Since patients in our study were included without specific rejection criteria, this may explain a lower success-rate.
PRF of the ABVN is an uncomplicated technique for energizing the vagal system. One can argue how specific this method is for VNS itself. In the ear also the trigeminal, facial, sympathetic, and spinal nerves (including the 2nd and 3rd cervical nerves) could be stimulated together1. Stimulating these nerves could start up the NST. However, they could also have additional effects. Exploration of the effects of stimulation each of these innervating nerves of the NST will increase our understanding of the ABVN projections and of tinnitus pathology.
Conclusion
Neuromodulation of the auricular branch of the vagal nerve is an uncomplicated remedy for tinnitus. In our research, 48% of tinnitus suffers who undertook PRF of the ABVN had less loudness of their tinnitus. An angle smaller than 3 degrees between the 2nd and 3rd cervical vertebrae on lateral radiograph can predict treatment success by PRF of the ABVN stimulation, with a chance of 64% on a successful outcome. This criterion adds to our knowledge of tinnitus heterogeneity and can help selecting future therapies, moving towards an individualized precisionmedicine approach for tinnitus.
The statements in our study are limited owing to its backward-looking quality. A prospective investigation, including a placebo-controlled double-blinded approach, is advised to affirm the outcome and our interpretations.
References
- Ouyang J, Pace E, Lepczyk L, Kaufman M, Zhang J, Perrine SA, e al. Blast-induced tinnitus and elevated central auditory and limbic activity in rats: a manganese-enhanced MRI and behavioral study. Sci Rep. 2017;7(1):4852.
- Bhatt IS. Prevalence of and risk factors for tinnitus and tinnitus-related handicap in a college-aged population. Ear Hear. 2018;39(3):517-26.
- Folmer RL, Griest SE. Chronic tinnitus resulting from head or neck injuries. Laryngoscope. 2003;113(5):821-7.
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101-4.
- Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, et al. Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med. 2018;10(422):eaal3175.
- De Ridder D, Langguth B, Vanneste S. Vagus nerve stimulation for tinnitus: A review and perspective. Prog Brain Res. 2021;262:451-67.
- Cederroth CR, Gallus S, Hall DA, Kleinjung T, Langguth B, Maruotti A, et al. Towards an understanding of tinnitus heterogeneity. Front Aging Neurosci. 2019;11:53.
- Cakmak YO. Concerning auricular vagal nerve stimulation: Occult neural networks. Front Hum Neurosci. 2019;13:421.
- Moffett J, Fray LM, Kubat NJ. Activation of endogenous opioid gene expression in human keratinocytes and fibroblasts by pulsed radiofrequency energy fields. J Pain Res. 2012:347-57.
- Beaumont E, Campbell RP, Andresen MC, Scofield S, Singh K, Libbus I, et al. Cervical vagus nerve stimulation augments spontaneous discharge in second-and higher-order sensory neurons in the rat nucleus of the solitary tract. Am J Physiol Heart Circ Physio. 2017;313(2):H354-67.
- Koning HM, van Hemert FJ. Pulsed Radiofrequency of the Vagal Nerve for Tinnitus-A Case-Study. Int Tinnitus J. 2021;25(2):172-5.
- Wu C, Martel DT, Shore SE. Increased synchrony and bursting of dorsal cochlear nucleus fusiform cells correlate with tinnitus. J Neurosci. 2016;36(6):2068-73.
- Zeng C, Shroff H, Shore S. Cuneate and spinal trigeminal nucleus projections to the cochlear nucleus are differentially associated with vesicular glutamate transporter-2. Neuroscience. 2011;176:142-51.
- Koehler SD, Pradhan S, Manis PB, Shore SE. Somatosensory inputs modify auditory spike timing in dorsal cochlear nucleus principal cells. Eur J Neurosci. 2011;33(3):409-20.
- Heeringa AN, Wu C, Chung C, West M, Martel D, Liberman L, et al. Glutamatergic projections to the cochlear nucleus are redistributed in tinnitus. Neuroscience. 2018;391:91-103.
- Koehler SD, Shore SE. Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS One. 2013;8(3):e59828.
- Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold's nerve (the auricular branch of the vagus nerve): A transganglionic HRP study in the cat. Brain Res. 1984;292(2):199-205.
- Yakunina N, Nam EC. Direct and transcutaneous vagus nerve stimulation for treatment of tinnitus: A scoping review. Front Neurosci. 2021;15:680590.
- Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275-99.
- Martins AR, Froemke RC. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci. 2015;18(10):1483-92.
- Mercante B, Ginatempo F, Manca A, Melis F, Enrico P, Deriu F. Anatomo-physiologic basis for auricular stimulation. Med Acupunct. 2018;30(3):141-50.
- Naser PV, Kuner R. Molecular, cellular and circuit basis of cholinergic modulation of pain. Neuroscience. 2018;387:135-48.
- Fast CD, McGann JP. Amygdalar gating of early sensory processing through interactions with locus coeruleus. J Neurosci. 2017;37(11):3085-101.
- Wichova H, Alvi SA, Shew M, Lin J, Sale K, Larsen C, Staecker H. Tinnitus perception in patients after vagal nerve stimulator implantation for epilepsy. Am J Otolaryngol. 2018;39(5):599-602.
- Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153:643-8.