Research Article - Biomedical Research (2017) Volume 28, Issue 3
Protective effect of bioflavonoid morin on Cadmium induced oxidative neuropathy
Song-hua Ma#, Ling-ling Zhang# and Qi-qun Jiang*Department of Neurology,the Second People’s Hospital of Nantong, Nantong, Jiangsu, China
#These authors contributed equally to this work
- *Corresponding Author:
- Qi-qun Jiang
Department of Neurology
The Second People’s Hospital of Nantong, China
Accepted on July 28, 2016
Abstract
Cadmium (Cd) a toxic heavy metal environmental pollutant imposes deleterious health consequences to human population. Reports suggest that rampant free radical generation and oxidative stress are the cardinal event in Cd induced neurotoxicity. Recently, therapeutic intervention using bioflavonoids gaining more attention to mitigate oxidative stress related pathologies. Thus the present study was scrutinized to study the bio efficacy of natural flavonoid Morin (MO) on Cd induced oxidative neurotoxicity. Cd intoxication (3 mg/kg; s.c) for 21 days displayed significant reduction in the activity of Acetylcholine Esterase (AChE), Monoaminooxidase (MAO) in the brain tissue. Further elevated lipid peroxidation and protein carbonyl content were also observed in Cd intoxicated rats. Meanwhile the activities of enzymic and non enzymic antioxidants were significantly reduced and the protein expression of apoptotic protein Caspase 3 was upregulated in Cd exposed rats. However, in the present study morin treatment (40 mg/kg) significantly restored the altered biochemical markers level to normalcy. Further, the protein expression of caspase 3 was significantly down regulated in morin administered rats. Thus, the neuroprotective potential of morin might be due to its effective antioxidant activity, anti-lipid peroxidative effect and through inhibition of apoptotic proteins.
Keywords
Morin, Cadmium, Heavy metals, Antioxidant, Flavonoid, Caspase 3.
Introduction
Heavy metal encompasses a series of elements like arsenic, cadmium and lead which elicits vulnerable toxic health hazards to human population. Betwixt the heavy metals, Cadmium (Cd) epitomized as the most noxious and carcinogenic element [1]. Cd is usually confronted by humans through waste from battery industries, metal coating, dyes and pigments, plastics products, resins, cigarette smoking and through dietary consumption [2-4]. Due to its extended half-life (15-30 years), Cd is accumulated in vital human tissues like kidney, liver , testis, bone and thus causes irreversible functional and structural damage to these cells [5,6]. Blood Brain Barrier (BBB) restricts the entry of foreign molecules but in the event of chronic minimal exposure of Cd, it disrupts the BBB integrity and adversely affects the brain [7]. Post BBB entry of Cd provokes neurotoxicity with array of clinical signs like behavioural changes, degeneration of neurons, and alteration in the neurochemical mediators [8]. Further, it has been shown that children exposed to Cd may develop learning disabilities and hyperactivity disorders [9]. Short term exposure of Cd may prelude to Parkinsonism disease in humans [10]. Brain is composed of high concentration of lipids which are vulnerable to oxidative free radical attack. During the Cd exposure, there exists a rampant generation of free radical precisely, superoxide radical which overture to induce Lipid Peroxidation (LPO) [11]. Furthermore, Cd mediated free radical generation in brain deplete the cellular antioxidant defence mechanism [12]. In addition, Cd interacts with mitochondrial sites and causes depletion in mitochondrial potential leading to diminished reduced glutathione levels [13]. Recently, plant derived flavonoid molecules gaining more attention for the mitigation of oxidative stress related diseases. In this scenario, morin a natural flavonoid ingredient found in wide array of Chinese medicinal plant displays potential anti-inflammatory and free radical scavenging activity [14]. Albeit, the neuroprotective effects of morin have been reported in various preclinical models its protective effect on Cd induced oxidative neurotoxicity is still under limelight. Hence, the present study was carried out to study the neuroprotective efficacy of morin against cadmium induced neurotoxicity in a murine model.
Materials and Methods
Drugs
Morin and cadmium chloride (CdCl2) were commercially obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Twenty four adult male Sprague-Dawley rats, aged 6-8 weeks and weighing 170-200 g, were maintained in temperature- and humidity-controlled animal houses under 12 h light/12 h dark cycles. The animals were fed standard food pellets and water ad libitum. All experiments were conducted in accordance with animal care guidelines approved by the Institutional Animal Ethics Committee (IAEC) of The Second People’s Hospital of Nantong University, Nantong, China.
Cadmium induced neurotoxicity
In the present study, the animals were divided into four groups (n=6) as follows,
Group I (Control)-Received saline (1.5 ml/kg; p.o) for 21 days.
Group 2 (Cd)-Injected with CdCl2 (3 mg/kg; s.c) for 21 days.
Group 3 (Mo)-Received morin alone (40 mg/kg; i.p) for 21 days.
Group 4 (Cd+Mo)-Treated with morin (40 mg/kg; i.p) 1hr before CdCl2 (3 mg/kg; s.c) injection and continued for 21 days.
After the study period, the animals were sacrificed by cervical decapitation. Then the whole brain was removed and washed with physiological saline and homogenized (Branson sonifier (250, VWR Scientific, USA) using ice-cold Tris-HCl buffer (50 mM, pH 7.4). Further, the homogenate was centrifuged at 3,000 rpm for 15 min at 4˚C. Finally, the supernatant was stored at -20˚C for analysis of various biochemical markers.
Estimation of acetyl cholinesterase activity (AchE)
Brain tissue level of Acetyl Cholinesterase (AChE) was estimated by Ellman et al. [14] method using acetylcholine iodide as a substrate. The method is based on the hydrolytic cleavage of acetylthiocholine iodide into thiocholine and butyric acid by AChE. Further, the formed thiocholine reacts with 5, 5′-dithiobis-2-nitrobenzoic acid to form a yellow colored product, 5- thio-2-nitrobenzoic acid. Then the color was measured spectrophotometrically (UV-1700, Shimadzu, Kyoto, Japan) at 412 nm.
Assessment of monoaminoxidase activity (MAO)
The brain level of MAO was estimated based on the procedure reported by Holt et al. [15]. The method is based on the enzymic oxidation of Benzylamine Hydrochloride (BAHC) to benzaldehyde. Briefly, to the 0.2 ml of supernatant, 0.4 ml of 0.1 M of phosphate buffer (pH 7.4), 1.3 ml distilled water and 0.1 ml of 0.1 M BAHC was added and incubated at room temperature for 30 mins. Then to the above reaction mixture 10% perchloric acid was added mixture and then centrifuged at 1,500 g for 10 min. Finally, the enzymic activity was measured at 280 nm and the units were expressed as μ moles of BAHC hydrolysed/min/mg of protein.
Measurement of lipid peroxidation and protein carbonyl content (PC)
Malondialdehyde (MDA), an effective marker of lipid peroxidation was estimated by the method of Ohkawa et al. [16]. The final colored end product was assayed spectrophotometrically at 532 nm. The MDA level was expressed as the n mole/g of tissue. The PC content in the brain homogenate was measured according to the procedure of Levine et al. [17]. The PC levels were expressed in terms of n mol/mg protein.
Estimation of enzymic antioxidant
The tissue levels of Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPx), Glutathione-S-Transferases (GST), Glutathione Reductase (GR) and Xanthine Oxidase (XO) in the brain tissue homogenate were measured using commercial kits (Nanjing, Jiancheng Co., China). The protein content was measured according to the procedure of Lowry et al. [18] using bovine serum albumin as a standard.
Evaluation of non enzymic antioxidants
Reduced Glutathione (GSH) was estimated by the method of Moron et al. [19], Vitamin C by Omaye et al. [20] and Vitamin E by the method of Desai [21].
Western Blot analysis of caspase-3 protein
Extraction of hepatic tissue proteins was done using RIPA lysis buffer kit (Applygen Technologies Inc., China). Then 20 μg of protein samples were resolved by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) for caspase-3. The blot was washed and incubated with goat anti-mouse IgG conjugated to peroxidase (Santa Cruz, USA). The binding of antibody was detected by ECL detection kit (Applygen Technologies Inc, China) and the protein bands were visualized using Gel Doc XR system (Bio-Rad, USA).
Statistical analysis
Results were expressed as mean ± SD (Standard Deviation). The differences between the group was done by one-way analysis of variance (Anova) followed by Tukey’s test to assess the significance. The p<0.05 were considered as statistically significant. The analysis was done by SPSS Statistical Software v13.
Results
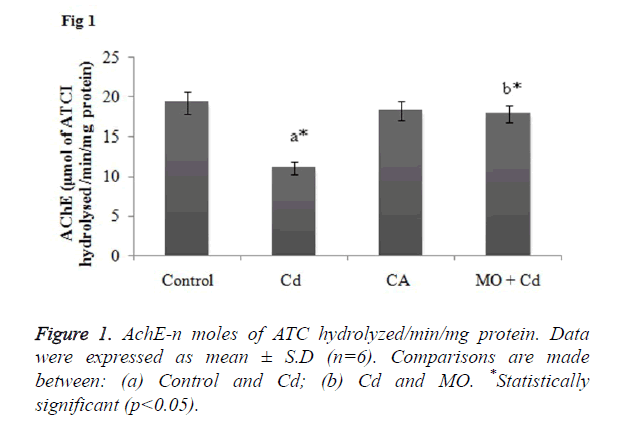
Effect of Cd and morin on AchE level in brain homogenate
AchE is a key enzymatic marker to assess the oxidative brain damage. So, decreased level of AchE in the brain may reflect a state of neurotoxicity. In the present study, Cd induced rats showed significant (p<0.05) reduction in the AchE activity as compared to the control rats. Whilst, treatment with morin effectively elevated the AchE activity in significant (p<0.05) manner as when compared to the Cd intoxicated group as shown in Figure 1.
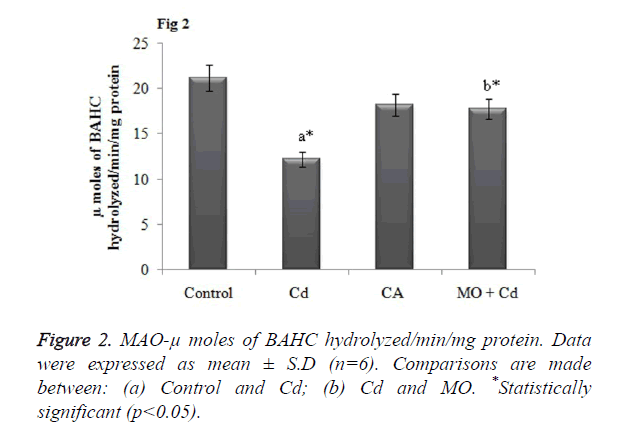
Effect of Cd and morin on MAO activity in brain
MAO is the cardinal enzyme involved in the breakdown of neuroactive amines for normal brain functions. Thus any changes in the MAO activity may alter or detoriate the brain functions. In our study, Cd intoxicated rats displayed dramatically decreased the MAO activity in brain tissue in a significant (p<0.05) manner as that of the control rats. However, morin intervention significantly (p<0.05) restored the altered MAO level to normalcy as shown in Figure 2.
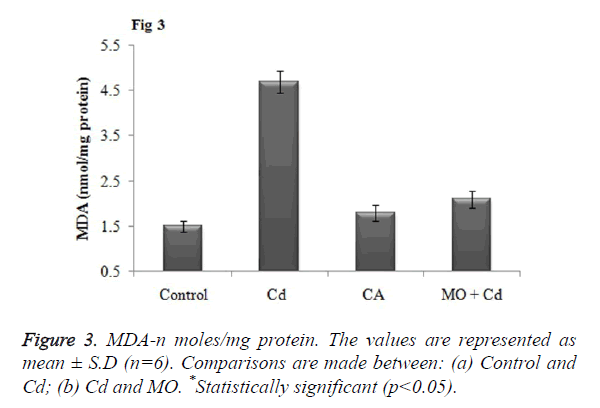
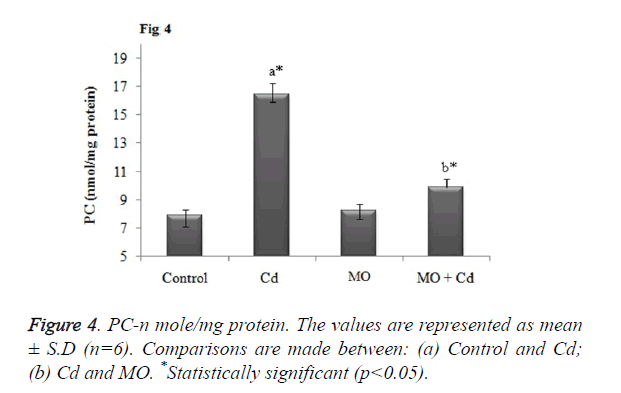
Effect of Cd and morin on lipid peroxidation and protein carbonyl content
Malondialdehyde (MDA), a prominent marker for lipid peroxidation was found to be significantly (p<0.05) elevated in brain tissue of Cd injected rats as when compared to the control. Meanwhile, treatment with morin significantly (p<0.05) restored the elevated MDA level to normalcy as when compared Cd intoxicated group as shown in Figure 3. Further, as a result of Cd induced protein oxidation, there was a significant (p<0.05) increase in the level of protein carbonyl content in Cd intoxicated as that of the control. Morin treatment significantly (p<0.05) diminished the PC content and restored the level to normalcy as shown in Figure 4.
Effect of Cd and morin on antioxidant enzymes activity
As a result of Cd provoked oxidative there was significant (p<0.05) decline in the activity of enzymic antioxidants, SOD, CAT, GPx, GST and XO in the brain tissue as that of the control animals. Whist, in morin treated group there was a significant elevation in the depleted antioxidant enzymes as compared to the Cd intoxicated group as shown in Table 1.
| Groups | SOD (U/mg of protein) | CAT (U/mg of protein) | GPx(n mol/min/mg protein) | GST(n mol/min/mg protein) |
|---|---|---|---|---|
| Control | 8.54 ± 0.34 | 5.35 ± 0.1 | 4.43 ± 0.13 | 6.59 ± 0.45 |
| Cd (3 mg/kg; s.c) | 5.19 ± 0.53a* | 2.76 ± 0.07a* | 2.38 ± 0.06a* | 4.18 ± 0.21a* |
| MO | 8.87 ± 0.14a*, b* | 4.75 ± 0.08a*, b* | 4.13 ± 0.18a*, b* | 5.62 ± 0.17b* |
| MO+Cd | 8.76 ± 0.04b* | 4.72 ± 0.14b* | 4.32 ± 0.15a*, b* | 5.43 ± 0.1b* |
| Groups | SOD (U/mg of Protein) | CAT (U/mg of Protein) | GPx(n mol/min/mg protein) | GST(n mol/min/mg protein) |
| Control | 8.54 ± 0.34 | 5.35 ± 0.1 | 4.43 ± 0.13 | 6.59 ± 0.45 |
| Cd (3mg/kg; s.c) | 5.19 ± 0.53a* | 2.76 ± 0.07a* | 2.38 ± 0.06a* | 4.18 ± 0.21a* |
| MO | 8.87 ± 0.14a*, b* | 4.75 ± 0.08a*, b* | 4.13 ± 0.18a*, b* | 5.62 ± 0.17b* |
| MO+Cd | 8.76 ± 0.04b* | 4.72 ± 0.14b* | 4.32 ± 0.15a*,b* | 5.43 ± 0.1b* |
| The data were represented as mean ± S.D. for six rats in each group. Comparisons are made between: (a) Control and Cd; (b) Cd and MO. *Statistically significant (p<0.05). | ||||
Table 1. Effect of morin on enzymic antioxidant levels in cadmium neurotoxicity.
Effect of Cd and morin on non-enzymic antioxidant markers
During oxidative brain damage, the elevated lipid peroxidation is concurrent with GSH depletion. Similarly, in our study level of non enzymic antioxidants (GSH, Vitamin C and Vitamin E) were significantly (p<0.05) decreased in rats intoxicated with Cd as that of the control group. Administration of morin significantly (p<0.05) boosted the decreased level of non enzymic antioxidants to normalcy as shown in Table 2.
| Groups | GSH | Vitamin C | Vitamin E |
|---|---|---|---|
| Control | 5.56 ± 0.48 | 2.82 ± 0.19 | 2.86 ± 0.24 |
| Cd (3mg/kg; s.c) | 2.65 ± 0.29a* | 1.69 ± 0.07a* | 1.34 ± 0.18a* |
| MO | 5.74 ± 0.35b* | 2.35 ± 0.12 | 2.76 ± 0.31 |
| MO+Cd | 6.62 ± 0.51b* | 2.76 ± 0.12b* | 2.23 ± 0.21b* |
| The data were represented as mean ± S.D. for six rats in each group. Comparisons are made between: (a) Control and Cd; (b) Cd and MO. *Statistically significant (p<0.05). Unit-GSH: n moles GSH/mg protein; Vitamin C: n moles/mg protein; Vitamin E: n moles/mg protein. |
|||
Table 2. Effect of morin on non-enzymic antioxidant levels in cadmium neurotoxicity.
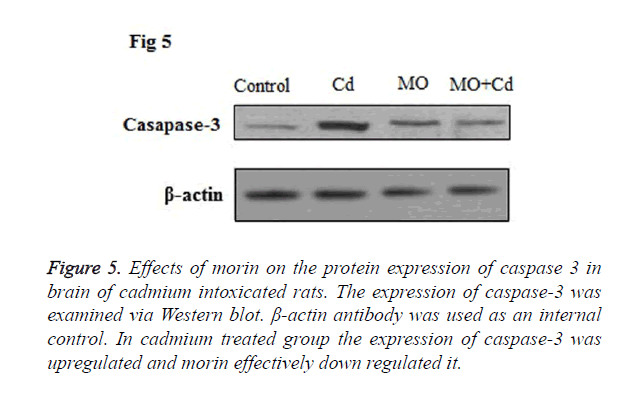
Effect of Cd and morin on caspase 3 protein expression
Caspase 3, an apoptotic mediator, orchestrates a pivotal role in the process of cell death. In our study, Cd insulted rats displayed upregulated protein expression of caspase-3 in the brain tissue as compared to the control. However, in morin treated rats the caspase 3 protein expressions was down regulated to normal level as shown in Figure 5.
Figure 5. Effects of morin on the protein expression of caspase 3 in brain of cadmium intoxicated rats. The expression of caspase-3 was examined via Western blot. β-actin antibody was used as an internal control. In cadmium treated group the expression of caspase-3 was upregulated and morin effectively down regulated it.
Discussion
Globally, environmental toxicants have been the imperative factor in the development and progression of CNS related disorders. Environmental pollution due to heavy metals causes deliberate noxious health consequences to humans and affects the quality of life. Cadmium (Cd) is encountered by humans through cigarette smoke, mining waste, fertilizers, sewage contamination in the agricultural land may lead to Cd uptake by plants for human nutrition [21]. Rampant generation of free radicals, oxidative stress and depletion of vital antioxidant molecules are the hallmark events in the pathophysiology of Cd provoked neurotoxicity. Further, preclinical reports elicits that, the impairment of brain function mediated by Cd is highly due to alteration in neurochemical transmission [22,23]. In CNS, Acetylcholine (ACh) is the prime cholinergic neurotransmitter involved in the transmission of impulse/ information between the neurons. ACh in the brain is biotransformed by the enzyme Acetylcholine Esterase (AchE) to choline and acetic acid. However, in the event of oxidative neurotoxicity AChE is inactivated leading to accumulation of ACh in the brain. Elevated AChE may prelude to toxic biological sequences like cholinergic hyperactivity, convulsion and status epilepsy [24]. The effect of Cd on AChE activity is concentration dependent, lower the concentration elevated AChE level and higher the concentration decreased AChE activity [25]. Reports suggest that Cd binds with AChE and alters the enzyme structure to produce unreactive enzyme species [26]. In the present study Cd exposed rats displayed significant reduction in the AChE activity and previous studies indicate that antioxidants are reported to exhibit anti AChE inhibitory activity [27,28]. Similarly, in our study morin intervention effectively restored the altered AChE level to normalcy which might be due to its antioxidant and free radical scavenging potential [29].
MAO have the ability to limit the action of many important neurotransmitters, oxidatively deaminate neurotransmitter and xenobiotic amines thereby setting up the basis of rapid repetitive response MAO plays an important role in catabolizing the neuroactive amines. The decreased level of MAO observed in the present study suggests that Cd has the potential to reach the CNS and impair its function. Meanwhile, treatment with morin significantly increased the level of MAO which might be due to enhancement of cholinergic function by morin.
The CNS is enriched with huge amounts of lipid and they are more prone to the attack of free radicals generated during oxidative neurotoxicity. The cardinal event in the Cd induced neurotoxicity is the ability to penetrate BBB and collocate in the brain to elicit lipid peroxidation. Cd induced lipid peroxidation is mediated by over generation of superoxide radical to form a toxic product Malondialdehyde (MDA) [30]. Further, Cd induced free radicals may cause oxidation deterioration of protein to form carbonyl groups, a toxic process during Cd mediated neurotoxicity [31]. In our study we observed an elevated level of MDA and protein carbonyl content in brain tissues of rats intoxicated with Cd. Treatment with morin elicited a significant reduction in elevated levels of MDA and protein carbonyl content [32].
Rampant lipid peroxidation process may affect the activity of non enzymic thiol antioxidants GSH which get utilized to minify the peroxidation of lipids. GSH, act as a prime scavenger of free radicals in wide array of oxidative stress pathology. Inactivation of GSH in one of the target mechanism in Cd induced neurotoxicity. Hepatic biotransformation of Cd lead to covalent binding to thiol related compounds like GSH and metallothioneins [33]. Cd specifically targets the cysteine residues of GSH and forms an inactive mercapeptide complex. Thus the structural modification hinders the antioxidant potential of GSH and thus makes the brain vulnerable to oxidative attack during Cd intoxication [34]. In corroboration to the above research reports, depleted GSH level was observed in our study. In addition, the other non enzymic antioxidants vitamin C and Vitamin E was found to be decreased in Cd insulted rats. Morin administration effectively improved the GSH, vitamin C and Vitamin E by reducing utilization of these molecules to counteract the free radical produced by Cd [35].
The brain antioxidant defensive system network encompasses a series antioxidant enzyme, SOD, CAT, GPx and GR. The primary response is elicited by SOD against the ROS and peroxidative stress. Whilst, the superoxide radical is generated initially in the ROS system and converted into H2O2 and molecular oxygen by catalase or GPx. Thus, vital tissues are more susceptibility to oxidative stress attack which may be attributed due to the diminished antioxidant levels [36]. In our study diminished activity of these antioxidant enzymes in the brain was observed in Cd exposed rats. The possible mechanism in Cd induced antioxidant enzyme depletion may be due to the interaction with SH groups, alteration in amino acid chain due to free radical mediated oxidation. Finally, these toxic changes lead to inactivation and loss of enzyme function [37]. Administration of morin refurbished the depleted antioxidant enzymes by inhibition of lipid peroxidation and oxidative stress [38].
Reports suggest that neuronal cell death elicited by Cd may be mediated through apoptosis or necrotic process depending on the target tissues and concentration. The apoptosis process induced by Cd is mitochondrial dependent in both the in vitro and in vivo conditions. Reports display that long term exposure of Cd results in the up regulation of apoptotic mediators (Bax, p53 and p21) and down regulation of Bcl-2 [39]. Further, among the apoptosis related caspase protein, Caspase 3 orchestrate a pivotal role in Cd provoked apoptosis [40]. In our present study, we found that caspase-3 was over-expressed in the brain of Cd exposed group, and morin intervention down regulated the protein expression to normalcy [41].
In conclusion, morin exerts the neuroprotective potential through restoration of AchE level, inhibition of lipid peroxidation, protein carbonylation, elevation of antioxidants and inhibition of apoptosis. Further, molecular studies are highly warranted to elucidate the mechanism of morin in mitigation of cadmium induced oxidative neurotoxicity.
References
- Brender JD, Suarez L, Felkner M, Gilani Z, Stinchcomb D, Moody K. Maternal exposure to arsenic, cadmium, lead and mercury and neural tube defects in offspring. Environ Res 2006; 101: 132-139.
- Hammond PB, Foulkes EC. Metal ion toxicity in man and animals. Metal Ions Biol Syst Marc Dek New York 1986; 157-200.
- El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid per-oxidation, blood haematology, biochemical parameters and semen quality of male rats-protective role of vitamin e and beta-carotene. Food Chem Toxicol 2004; 42: 1563-1571.
- de Souza PF, Diamante MA, Dolder H. Testis response to low doses of cadmium in wistar rats. Int J Exp Pathol 2010; 91: 125-131.
- Fowler BA. Monitoring of human populations for early markers of cadmium toxicity- a review. Toxicol Appl Pharmacol 2009; 238: 294-300.
- Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure and health outcomes. Environ Health Perspect 2010; 118: 182-190.
- Murphy VA. Cadmium acute and chronic neurological disorders. Mineral and Metal- Neurotoxicol Boca Raton 1997; 229-240.
- Renugadevi J, Miltonprabu S. Protective role of alpha tocopherol and ascorbic acid against cadmium induced neurotoxicity in rats. Int J Med Sci 2009; 2: 11-17.
- Wang B, Du Y. Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013: 898034.
- Okuda B, Iwamoto Y, Tachibana H, Sugita M. Parkinsonism after acute cadmium poisoning. Clin Neurol Neurosurg 1997; 99: 263-265.
- EL-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood haematology, biochemical parameters and semen quality of male rats-Protective role of vitamin E and beta-carotene. Food Chem Toxicol 2004; 42: 1563-1571.
- Shukla GS, Hussain T, Srivastava RS, Chandra SV. Glutathione peroxidase and catalase in liver, kidney, testis and brain regions of rats following cadmium exposure and subsequent withdrawal. Ind Health 1989; 27: 59-69.
- Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. Distinctive antioxidant and antiinflammatory effects of flavonols. J Agri Food Chem 2006; 54: 9798-9804.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88-95.
- Holt A, Sharman DF, Baker GB, Palcic MM. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal Biochem 1997; 244: 384-392.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
- Levine RL, Garland D, Oliver CN, Amic A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Method Enzymol 1990; 186: 464-478.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folins phenol reagent. J Biol Chem 1951; 193: 265-275.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979; 582: 67-78.
- Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol 1979; 62: 3-8.
- Desai DI. Vitamin E analysis methods for animal tissues. Methods Enzymol 1984; 105: 138-147.
- Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 2009; 238: 201-208.
- Antonio MT, Corredor L, Leret ML. Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol Lett 2003; 143: 331-340.
- Tsakiris S, Angelogianni P, Schulpis KH, Starridis JC. Protective effect of l-phenylalanine on rat brain acetylcholinesterase inhibition induced by freeradicals. Clin Biochem 2000; 33: 103-106.
- Olney JW, Collins RC, Sloviter RS. Exotoxic mechanisms of epileptic brain damage. Adv Neurol 1986; 44: 857-877.
- Carageorgiou H, Tzotzes V, Pantos C, Mourouzis C, Zarros A, Tsakiris S. In vivo and in vitro effects of cadmium on adult rat brain total antioxidant status, acetylcholinesterase, (Na+, K+)-ATPase and Mg2+-ATPase activities: protection by L-cysteine. Basic Clin Pharmacol Toxicol 2004; 94: 112-118.
- Tomlinson G, Mutus B, McLennan I. Activation and inactivation of acetylcholinesterase by metal ions. Can J Biochem 1981; 59: 728-735.
- Dong C. Protective effect of proanthocyanidins in cadmium induced neurotoxicity in mice. Drug Res 2015; 65: 555-560.
- Abdel Moneim AE, Bauomy AA, Diab MM, Shata MT, Al-Olayan EM, El-Khadragy MF. The protective effect of Physalis peruviana L. against cadmium-induced neurotoxicity in rats. Biol Trace Elem Res 2014; 160: 392-399.
- Remya C, Dileep KV, Tintu I, Variyar EJ, Sadasivan C. Design of potent inhibitors of acetylcholinesterase using morin as the starting compound. Front Life Sci 2012; 6: 107-117.
- Adefegha SA, Omojokun OS, Oboh G, Fasakin O, Ogunsuyi O. Modulatory effects of ferulic acid on cadmium-induced brain damage. J Evid Based Complementary Altern Med 2015; 1-6.
- Eneman JD, Potts RJ, Osier M, Shukla GS, Lee CH, Chin JF. Suppressed oxidant-induced apoptosis in cadmium adapted alveolar epithelial cells and its potential involvement in cadmium carcinogenesis. Toxicology 2000; 147: 215-228.
- Subash S, Subramanian P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol Cell Biochem 2009; 327: 153-161.
- Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin 1, thiore-doxin 2 and glutathione by metal ions. Free Radic Biol Med 2006; 40: 138-145.
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part1: mechanisms involved in metal induced oxidative damage. Curr Top Med Chem 2001; 1: 529-539.
- Al-Numair KS, Chandramohan G, Alsaif MA, Veeramani C, El Newehy AS. Morin, a flavonoid, on lipid peroxidation and antioxidant status in experimental myocardial ischemic rats. Afr J Tradit Complement Altern Med 2014; 11: 14-20.
- Sharma B, Singh S, Siddiqi NJ. Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int 2014; 2014: 640754.
- Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis-a review. Toxicol 2003; 192: 95-117.
- Zhang R, Kang KA, Piao MJ, Maeng YH, Lee KH, Chang WY, You HJ, Kim JS, Kang SS, Hyun JW. Cellular protection of morin against the oxidative stress induced by hydrogen peroxide. Chem Biol Interact 2009; 177: 21-27.
- Agnihotri SK, Agrawal U, Ghosh I. Brain most susceptible to cadmium induced oxidative stress in mice. J Trace Elem Med Biol 2015; 30: 184-193.
- Nazima B, Manoharan V, Miltonprabu S. Cadmium induced cardiac oxidative stress in rats and its attenuation by GSP through the activation of Nrf2 signaling pathway. Chem Biol Interact 2015; 242: 179-193.
- Wei Z, He X, Kou J, Wang J, Chen L, Yao M, Zhou E, Fu Y, Guo C, Yang Z. Renoprotective mechanisms of morin in cisplatin-induced kidney injury. Int Immunopharmacol 2015; 28: 500-506.