Research Article - Biomedical Research (2018) Artificial Intelligent Techniques for Bio Medical Signal Processing: Edition-II
Protective effect and treatment of cucurbitacin B on mice with sepsisassociated acute kidney injury
Dongming Zheng1, Shanshan Zhang2, Dejian Hu3 and Xueheng Zhou3*
1Department of Pharmacy, Qingzhou people's Hospital, Weifang, PR China
2Department of Pharmacy, Anqiu People's Hospital, Weifang, PR China
3Department of Medicine, Affiliated Hospital of Weifang Medical University, Weifang, PR China
- *Corresponding Author:
- Xueheng Zhou
Department of Medicine
Affliated Hospital of Weifang Medical University, PR China
Accepted on May 23, 2017
DOI: 10.4066/biomedicalresearch.29-16-2258
Visit for more related articles at Biomedical ResearchAbstract
Ground Penetrating Radar (GPR) is vastly utilized for non-invasive study of man-made structures, particularly for determining depth of pipes which are buried under the ground. However, shallow objects might obscure GPR raw data which is reflected from deeper ones. The current work suggests a signal processing method known as the parallel lifting-based Canonical Signed Digit (CSD) 2D Discrete Wavelet Transform (DWT) infrastructure is suggested to realize a multiplier less architecture with lesser hardware complexity, smaller area and power consumption. The flipping structure of the lifting scheme can be recognized to reduce the longest critical path. CSD based structure is demonstrated with only adders and free of multipliers. A stripe based scanning method is adopted in order to achieve an efficient memory. JPEG 2000 lossy 9/7 filter is structured and the same scanning method with CSD is used to design 6/10 filter to contribute the evidence for the proposed methodology. In the existing method, memory efficiency is achieved with less speed. The new architecture is proposed using CSD multiplier with more speed. The filter coefficients are multiplied by 256 and are converted to integer form and then CSD representation is considered. The suggested architecture provides multiplier-less infrastructure for DWT utilizing CSD. This infrastructure is appropriate for high speed online applications with less area and power consumption. For an N× (N+1) image, the suggested CSD based lifting infrastructure utilizes solely 3N temporal memory, 2S transposition memory as well as Tm+Ta critical path.
Keywords
Sepsis, AKI, Inflammatory factors, Cucurbitacin B.
Introduction
Acute kidney injury (AKI) exists or happens worldwide and sepsis has long been recognized as a foremost precipitant of AKI. To date, no therapeutic measures are available to prevent or treat sepsis-associated AKI due to limited understanding of pathophysiologic mechanisms. As a natural traditional Chinese medicine (TCM), cucurbitacin B has a variety of pharmacological effects such as anti-virus and antiinflammatory. A great diversity of disease models has witnessed its obvious anti-inflammatory effect, with good curative effect and moderate drug property. However, the antiinflammatory effect of cucurbitacin B in sepsis-associated AKI model hasn’t yet reported.
In recent years, the death is occurring commonly due to sepsis and septic shock which is a thoughtful clinical picture affected by the systemic response against infection along with dysfunction of organ at particular stage. Finding of sepsis is ambiguous. To analyze medically, there is a need of support like leucocyte count, body temperature and presentation of bacterial antigens in plasma and body fluids. Recent awareness is to predict and treat the sepsis early though there is a positivity in blood culture. AKI is a clinical disorder which lowers glomerular filtration rate, growth of nitrogenous waste products like urea and creatinine and syndrome of acid-base and fluid and electrolyte balances. Among serious care patients, 35% are affected by AKI because more than 50% of AKI patients are affected by sepsis and septic shock. Sepsis related to AKI consists of the mortality rate ranging from 20.9% and 56.8% based on force of injury. Constant increase of AKI and sepsis occurrence leads to serious clinical and public health problem and so predicting early and treating lowers the risks [1].
The sepsis occurred when the initial, suitable host reply to an infection leads to improve and then deregulated. First it is necessary to find the sepsis induced AKI and to select the suitable therapeutic modality since it has very high mortality rates. Most commonly affected organ due to sepsis is the kidney which holds a high risk of mortality. In case of sepsis, the difficult one is the pathophysiology of AKI which comprises of intrarenal hemodynamic changes, endothelial dysfunction, infiltration of inflammatory cells in the renal parenchyma, intraglomerular thrombosis, and block of tubules with necrotic cells and debris. By pro and anti-inflammatory mechanisms [2], the sepsis-induced immune replies are contain the activation in progressive way.
This study adopted the caecal ligation puncture to establish the mouse model of sepsis-associated AKI, where 120 male KM mice were randomly divided into 5 groups, namely sham operation control group, sepsis model group, high cucurbitacin B (5 mg/kg.bw), medium cucurbitacin B (2 mg/kg.bw) and low cucurbitacin B (1 mg/kg.bw) dosage groups. The protective effect and possible mechanism of cucurbitacin B on sepsisassociated AKI was investigated by observing and comparing the levels of creatinine (Cre), blood urea nitrogen (BUN), TNF-a and IL-6 in serum at different time after operation on mice, calculating the organ coefficient after kidney weighing, and observing the pathological changes of renal tissue by HE staining. The results showed that cucurbitacin B could effectively reduce the contents of the Cre and BUN, significantly inhibited the inflammatory response and reduced renal pathological damage, thus protecting the sepsis– associated AKI at a high level. This study provides a solid foundation and theoretical basis for the clinical application of cucurbitacin B in patients with sepsis–associated AKI.
Preface
Sepsis is commonly a systemic inflammatory response syndrome (SIRS) triggered by an infection, which is characterized by excessive inflammatory response and uncontrolled release of inflammatory mediators. The sepsis may develop rapidly and end up with septic shock and multiple organ dysfunction syndrome (MODS), which seriously endangers the lives of patients, constituting one of the most common causes of death for critically ill patients with severe infection, burns, severe trauma, major surgery, etc. [3] The incidence of sepsis or septic shock is high and increasing with years all over the world. A 22-year retrospective analysis of hospitalization records in the USA found an 8.7% annual increase for a sepsis diagnosis [4,5]. A domestic epidemiological survey also revealed that incidence of severe sepsis and septic shock in the ICU was 37.3% and the mortality rate was even up to 28.7% [6].
Essentially, sepsis is the body's response to infection. During its process of pathology and physiology, MODS develops as the last stage, with kidney being one of the most commonly targeted organs. It’s reported that sepsis is the most common contributing factor for the development of AKI. About 26% to 50% of the AKI was associated with sepsis [7,8]. Conversely, the decline of glomerular filtration rate in the early stages of AKI led to significant increasing of the mortality of patients with sepsis [9]. Therefore, AKI, as the complication of sepsis, not only poses difficulties for the effective intervention, increasing patients’ mortality, but also consumes a huge medical resources and expenses. In addition, the mechanism in pathology and physiology of sepsis-associated organ dysfunction is still unclear, thus, deep investigation of pathophysiological process and mechanism of sepsis associated AKI, and discovery of new therapies and efficacious drugs is of great significance for lowering morbidity and mortality in patients with sepsis. At present, supportive care is adopted for severe sepsis-associated AKI, including antibiotics, diuretics and vasoactive drugs, etc. In recent years, given the advantages of effectivity, moderation and small side effects in TCM, some TCM therapies have been studied, for example, the combination of Xuebijing (Purify blood) injection, Ulinastatin along with Shenmai injection with rhubarb preparations, etc.
Cucurbitacin, a natural herbal extract, is a class of highly oxidized bitter or sweet tetracyclic triterpenoid compounds extracted from various plants of cucurbitaceae and other genera. Cucurbitacin is divided into 12 categories based on different chemical structures. Among them, cucurbitacin B is the most abundant in the family, and has extensive pharmacological activities such as hepatoprotective, antiviral, antibacterial, anti-inflammatory, anti-cancer, etc., [10-16] However, study on cucurbitacin B about its regulation and mitigation on sepsis-associated AKI and inflammatory response is still blank. Therefore, by adopting caecal ligation puncture to establish the mouse model of sepsis-associated AKI for observing the contents of Cre, BUN, TNF-a and IL-6 in the blood at different time after operation on mice, calculating the organ coefficient after kidney weighing, and observing the pathological changes of renal tissue by HE staining, this study aimed to investigate the mechanism of cucurbitacin B on sepsis-associated AKI and evaluate its preventive and therapeutic effect on sepsis-associated AKI, thus finding a new effective method for clinical treatment of sepsis-associated AKI.
Materials and Methods
Experimental animals
Adult male Kunming (KM) mice aged 16~18 weeks, weighing 25 to 30 g, were purchased from Medical Experimental Animal Center of Guangdong Province and were housed in a pathogenfree SPF animal room with a 12h light and 12h dark cycle at room temperature (24 ± 2°C) and a relative humidity of 40-70%, free access to water and food. The research was conducted in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the United National Institutes of Health.
Animal grouping, model construction and postoperative administration
Mice were housed 3 days before the experiment for the adaptation in new environment. These Male KM mice (n=120) were randomly divided into 5 groups, namely sham operation control group (Sham), sepsis model group (Model), high cucurbitacin B (CB-H) (5 mg/kg.bw), medium cucurbitacin B (CB-M) (2 mg/kg.bw) and low cucurbitacin B (CB-L) (1 mg/ kg.bw) dosage groups. These mice fasted but had free access to water 12 h before operation. Cecal ligation puncture was used to establish the mouse model of sepsis-associated AKI. General anesthesia was induced by intraperitoneal injection of 10% hydrated chloric acid 0.3 ml/100 g.bw. Cecum was removed from the abdominal cavity, followed by the separation of mesenteric vascular. Then the middle part between caecal terminal and the ileocecal valve was ligated. The central section in the ligation was punctured with a little feces squeezed out before putting the cecum back. Finally, surgical incision was sutured accordingly. While for the sham operation control group, no surgical treatment was given except that the abdominal cavity was opened to locate the cecum and then just put it back for suture. After operation, all agents were injected intraperitoneally with cucurbitacin B diluted with 0.1% DMSO to 3 concentrations: 5 mg/ml (for CB-H dosage group), 2 mg/ml (for CB-M dosage group) and 1 mg/ml (for CB-L dosage group). 0.1% DMSO was given to sham operation control group and sepsis model group at the same volume.
Specimen collection
Eight mice were randomly selected at the time points (6 h, 12 h, 24 h) after model establishment and administration. These mice were anesthetized intraperitoneally with 1.5% pentobarbital sodium at 0.1 ml/20 g.bw. Next, 2 ml of blood were taken from the abdominal aorta after the laparotomy for test in Cre, BUN, TNF-a and IL-6. Tissues from both kidneys were weighed and stained with hematoxylin-eosin (HE) after treated with formalin, then mounted for histopathologic examination.
Analysis on levels of Cre and BUN and organ coefficient
Cre and BUN levels were measured with automatic biochemical analyzers (Mindray Biomedical, Shenzhen). All mice were sacrificed painlessly, followed by gross observation and weighting of kidney for organ coefficient analysis. The formula is as below:
Organ coefficient (%)=Organ mass/Body mass (after fasting) × 100.
ELISA assessment for levels of inflammatory factors in serum
Serum stored in a refrigerator at -80°C was thawed for the determination of TNF-a and IL-6 in strict accordance with the instructions of the ELISA kit (TNF-a, IL-6).
Staining of renal tissue with HE
One side of the renal tissue was treated with 4% paraformaldehyde for 24 h, dehydrated step by step in a graded ethanol series (70-100%) and fixed with xylene for 30 min. Then it was embedded in paraffin at low melting point 52 °C, cut into slices and stained with HE. Finally, the pathological changes of renal tissue were observed under light microscope.
Statistical methods
All statistical analysis was performed with SPSS for Windows 17.0 software and all the data for measurement were expressed as mean ± standard deviation (X ± SD). One-way analysis of variance (ANOVA) was adopted for the comparison of the mean between groups and t test for that between two samples when mean difference was statistically significant, p<0.05.
Results
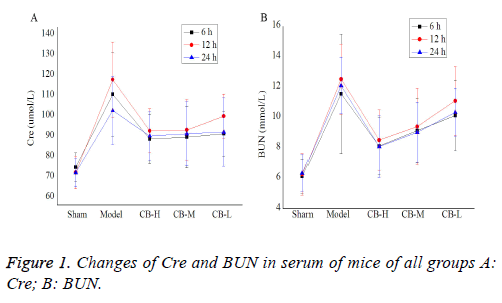
Changes of Cre and BUN in serum of mice: Cre and BUN of all groups at different time were shown in Table 1. Compared with the control group, mice in the model group were observed AKI at some level, with significant increase of Cre and BUN, which reached the highest level at 12 h. The difference between these two groups was statistically significant (P<0.05). The comparison between dosage groups and model group indicated that different doses of cucurbitacin B significantly lowered the levels of Cre and BUN in serum (P<0.05), and the efficacy is dose-dependent (Figure 1).
| Groups | Sham | Model | CB-H | CB-M | CB-L | |
|---|---|---|---|---|---|---|
| Cre µmol/L | 6 h | 73.61 ± 7.13 | 109.46 ± 20.55* | 87.49 ± 12.08# | 88.47 ± 15.13* | 90.11 ± 11.16* |
| 12 h | 71.01 ± 8.00 | 116.73 ± 18.46* | 91.55 ± 10.76*# | 92.03 ± 14.83*# | 98.73 ± 10.86* | |
| 24 h | 70.98 ± 6.89 | 101.49 ± 16.72* | 89.03 ± 11.86*# | 90.11 ± 15.77* | 90.97 ± 16.79 | |
| BUN mmol/L | 6 h | 6.13 ± 1.13 | 11.56 ± 3.94* | 8.13 ± 1.86# | 9.14 ± 2.13* | 10.13 ± 2.31* |
| 12 h | 6.27 ± 1.35 | 12.53 ± 2.31* | 8.52 ± 1.99*# | 9.41 ± 2.51*# | 11.10 ± 2.25* | |
| 24 h | 6.35 ± 1.20 | 12.09 ± 1.86* | 8.09 ± 2.01# | 9.03 ± 1.9*8 | 10.34 ± 1.58* | |
Note: * indicated when compared with the sham operation control group, p<0.05; # indicated when compared with the sepsis model group, p<0.05.
Table 1. Changes of Cre and BUN in serum of mice.
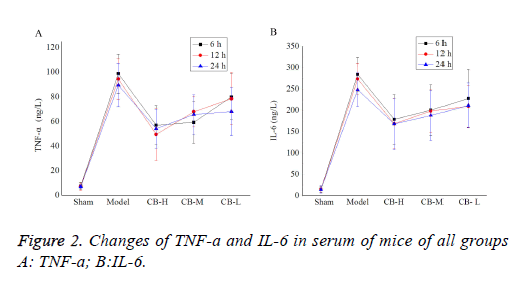
Changes of serum TNF-a and IL-6 in mice
Changes of cytokines, TNF-α and IL-6 of all groups were assessed by ELISA. As shown in Table 2, in contrast with the sham operation control group, significant inflammatory reaction in kidney was observed in the model group, showing evident rise of TNF-a and IL-6 in serum (P<0.05), peaked at 6 h. Whereas, the comparison between dosage groups and model group suggested that cucurbitacin B can inhibit inflammatory reaction of the kidney, and different doses of cucurbitacin B variably lowered the levels of TNF-a and IL-6 in serum, and the efficacy is dose-dependent (Figure 2).
| Groups | Sham | Model | CB-H | CB-M | CB-L | |
|---|---|---|---|---|---|---|
| TNF-a ng/L | 6 h | 7.52 ± 2.90 | 98.81 ± 15.81* | 56.94 ± 15.96*# | 59.28 ± 17.03* | 80.02 ± 18.55* |
| 12 h | 7.02 ± 3.01 | 94.46 ± 16.73* | 49.51 ± 21.18*# | 67.99 ± 12.45*# | 78.57 ± 21.16* | |
| 24 h | 6.93 ± 1.44 | 89.46 ± 17.49* | 54.19 ± 15.82*# | 65.73 ± 16.07*# | 68.09 ± 19.39* | |
| IL-6 ng/L | 6 h | 15.19 ± 6.97 | 284.34 ± 39.46* | 179.36 ± 58.19*# | 200.78 ± 59.12* | 228.14 ± 67.39*# |
| 12 h | 14.85 ± 7.91 | 273.34 ± 36.31* | 169.18 ± 59.30*# | 198.64 ± 49.58*# | 209.74 ± 48.48* | |
| 24 h | 15.01 ± 8.81 | 248.65 ± 38.97* | 168.60 ± 60.18*# | 188.66 ± 58.86*# | 211.86 ± 52.38* | |
Note: * indicated when compared with the sham operation control group, p<0.05; # indicated when compared with the sepsis model group, p<0.05.
Table 2. Changes of TNF-a and IL-6 in serum of mice.
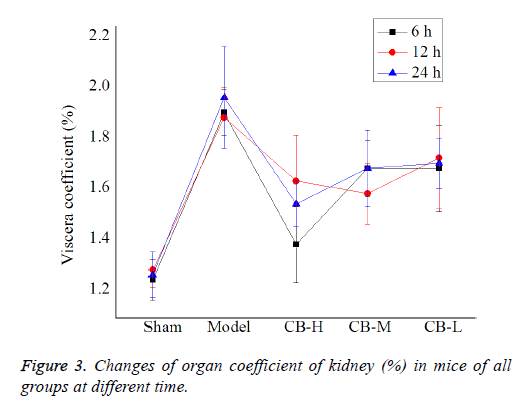
Change of organ coefficient of kidney in mice
Table 3 and Figure 3 depict organ coefficient of kidney of each group at different time. Compared with the sham operation control group, organ coefficient of kidney of the model group was significantly high (P<0.05), which suggested serious AKI in the model group. Compared with the model group, organ coefficient of kidney of cucurbitacin B dosage groups decreased slightly, with some results statistically significant (P<0.05). This means that cucurbitacin B can inhibit the inflammatory reaction in kidney of mice. The results indicated that all the three dosages of cucurbitacin B can significantly lower the organ coefficient of kidney (P<0.05), and the efficacy is concentration-related.
| Groups | Sham | Model | CB-H | CB-M | CB-L |
|---|---|---|---|---|---|
| Organ coefficient of kidney 6 h | 1.25 ± 0.08 | 1.91 ± 0.09* | 1.39 ± 0.15# | 1.69 ± 0.11* | 1.69 ± 0.17* |
| Organ coefficient of kidney 12 h | 1.29 ± 0.07 | 1.89 ± 0.12* | 1.64 ± 0.18*# | 1.59 ± 0.12# | 1.73 ± 0.20* |
| Organ coefficient of kidney 24 h | 1.27 ± 0.09 | 1.97 ± 0.20* | 1.55 ± 0.09# | 1.69 ± 0.15*# | 1.71 ± 0.10* |
Note: *indicated when compared with the sham operation control group, p<0.05; # indicated when compared with the sepsis model group, p<0.05.
Table 3. Changes of organ coefficient of kidney (%) in mice.
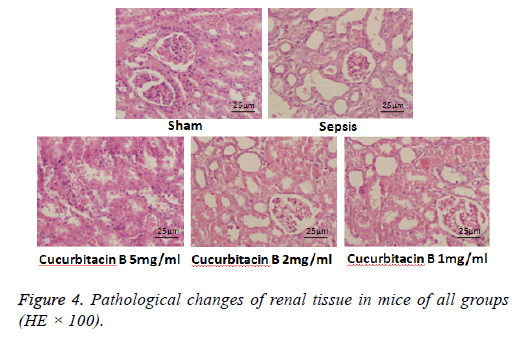
The pathological changes of renal tissue at different time
Histopathological examination revealed that structure of the renal tissue in the sham operation control group was clear, renal tubules and glomeruli were normal, renal tubular epithelial cells showed no signs of degeneration, atrophy, swell, necrosis or inflammatory infiltration, and no expansion of the lumen was examined, either. But different degrees of injury in the model group and cucurbitacin B dosage groups was observed, such as telangiectasia and hyperemia in glomeruli, edema, granular degeneration and vacuolar degeneration in renal tubular epithelial cell, luminal narrowing and neutrophil infiltration.
What worth noticing is that in cucurbitacin B dosage groups, in contrast with the sepsis model group, the degree of glomerular damage was less, edema, granular degeneration, vacuolar degeneration etc. in renal tubular epithelial cell were significantly reduced, and neutrophil infiltration also decreased (Figure 4).
Discussion
Sepsis is a disease with multiple factors involved, which commonly triggers the inflammatory reaction in the systemic immune system and may eventually develop into multiple organ failure [17]. Many clinical trials have shown that up to half of patients with sepsis will suffer from AKI, which, as a complication of sepsis, will increase the mortality significantly [7,8]. In spite of the increasing research in this direction and improvement of medical technology, death rate of sepsisassociated AKI has not decreased in the past 30 years, and the effective treatment is still scarcely found. During the last 10 years, medical research on the understanding of sepsis has made great progress and some achievements in pathogenesis and cell biology have been made. Despite the complexity of pathophysiological development in sepsis–associated AKI, numerous studies have shown that AKI is triggered by multiple factors, such as the mass production and release of inflammatory mediators, change in renal hemodynamic, abnormality of coagulation function, immune-induced injury and apoptosis as well as pathophysiological changes in a few systems and organs. Among them, the start-up of "cascade effect" following the incontrollable inflammatory mediators plays a particularly important role [18-22].
When excessive inflammatory reaction caused by infection in patients with sepsis occurs, inflammatory response disorders arise, and mononuclear cells and glomerular mesangial cells stimulated by endotoxin secrete a variety of pro-inflammatory factors such as IL-1, IL- 6, TNF-α and platelet activating factors. The bond of IL-1β [23], TNF-α [24,25] and IL-6 [26] and their respective receptors causes apoptosis in glomerular endothelial cell and infiltration in glomerular neutrophil, which, stimulates synthesis and secretion of active substances in mesangial cell for the involvement of local tissue damage and regulation. On the other hand, causes retention of neutrophils and mononuclear cells in glomeruli, resulting in free oxygen radicals and other tissue damage. Meanwhile, the activation of complements prompts the release of a variety of vasoactive substances and inflammatory mediators, combining into a gradual amplification of the inflammatory cascade and posing as a key factor for aggravated sepsis-associated AKI [27,28]. Therefore, focus should be placed on seeking new and effective drugs and measures that can inhibit the inflammation of sepsis-associated AKI, thereby reducing the mortality.
Cucurbitacin is a kind of natural TCM ingredient, a tetracyclic triterpene compound, extracted from cucurbitaceae and other genera. Cucurbitacin is divided into 12 categories according to the position of tetracycloalkyl on its chemical structure, with cucurbitacin B being the most abundant in content [10]. Previous studies have shown that cucurbitacin B has a variety of pharmacological biological activity and effects, such as anti Cucurbitacin is a kind of natural TCM ingredient, a tetracyclic triterpene compound, extracted from cucurbitaceae and other genera. Cucurbitacin is divided into 12 categories according to the position of tetracycloalkyl on its chemical structure, with cucurbitacin B being the most abundant in content [10]. Previous studies have shown that cucurbitacin B has a variety of pharmacological biological activity and effects, such as antivirus, anti-inflammatory and anti-cancer, thus extensively used in prevention and treatment of hepatitis and liver cancer [10-12]. Recent studies have shown that cucurbitacin B can inhibit imiquimod-induced inflammatory reaction in keratinocytes and the development of psoriasis in psoriasis models [29]. Cucurbitacin B was also found to inhibit LPSinduced macrophage inflammatory reaction and that its antiinflammatory effect was dependent on the activation and inducing of heme oxygenase-1 expression by Nrf2 [14]. These data suggest that with immunomodulatory and antiinflammatory effects, cucurbitacin B may be able to function as an effective TCM active substance for the treatment and prevention of inflammation.
This study successfully established mice model of sepsisassociated AKI through the caecal ligation puncture, where cucurbitacin B was used as a therapeutic drug to explore its role in treating renal tissue in flammation. The results showed that high and medium dose of cucurbitacin B can significantly lower the organ coefficient of kidney of mice, reduce edema, granular degeneration and vacuolar degeneration in renal tubular epithelial cell, and decrease neutrophil infiltration. Moreover, different doses of cucurbitacin B can decrease levels of TNF-a and IL-6 in serum in varying degrees. These suggest that cucurbitacin B can effectively inhibit the inflammatory reaction and reduce renal pathological damage, and thus protect sepsis-associated AKI. The possible mechanism is that cucurbitacin B may inhibit inflammatory reaction in sepsis and reduce sepsis-associated AKI by restraining the secretion of key inflammatory factors, like serum TNF-a and IL-6. Therefore, this study preliminarily indicates that for treating patients with sepsis, cucurbitacin B can be used as an alternative, since it can reduce AKI and improve the condition without obvious side effects. However, the specific molecular mechanism of cucurbitacin B in inhibiting inflammatory reaction during pathological process of sepsis-associated AKI remains further study and verification in vitro and in vivo.
References
- Bilgili B, Haliloglu M, Cinel I. Sepsis and Acute Kidney Injury. Turk J Anaesthesiol Reanim 2014; 42: 294-301.
- Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol 2011; 22: 999-1006.
- Dellinger RP. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580-637.
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546-1554.
- Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41:1167-1174.
- Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, Ai Y, Xu Y, Liu D, An Y, Wu D, Sun R, Li S, Hu Z, Cao X, Zhou F, Jiang L, Lin J, Mao E, Qin T, He Z, Zhou L, Du B; China Critical Care Clinical Trials Group. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 2014; 9: e107181.
- Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 2008; 12: R47.
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H. Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34: 344-353.
- Honore PM, Jacobs R, Hendrickx I, Bagshaw SM, Joannes-Boyau O, Boer W, De Waele E, Van Gorp V, Spapen HD. Prevention and treatment of sepsis-induced acute kidney injury: an update. Ann Intensive Care 2015; 5: 51.
- Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 2005; 22: 386-399.
- Kapoor S, Cucurbitacin B and its rapidly emerging role in the management of systemic malignancies besides lung carcinomas. Cancer Biother Radiopharm 2013; 28: 359.
- Alghasham AA. Cucurbitacins - a promising target for cancer therapy. Int J Health Sci (Qassim) 2013; 7: 77-89.
- Sun Y. Synergistic effect of cucurbitacin B in combination with curcumin via enhancing apoptosis induction and reversing multidrug resistance in human hepatoma cells. Eur J Pharmacol 2015; 768: 28-40.
- Kim M. Cucurbitacin B inhibits immunomodulatory function and the inflammatory response in macrophages. Immunopharmacol Immunotoxicol 2015; 37: 473-480.
- Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 2005; 22: 386-399.
- Jayaprakasam B, Seeram NP, Nair MG. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett 2003; 189: 11-16.
- Mårtensson J, Bellomo R. Sepsis-Induced Acute Kidney Injury. Crit Care Clin 2015; 31: 649-660.
- Haase-Fielitz A. Genetic polymorphisms in sepsis- and cardiopulmonary bypass-associated acute kidney injury. Contrib Nephrol 2007; 156: 75-91.
- Cho KC. Survival by dialysis modality in critically ill patients with acute kidney injury. J Am Soc Nephrol 2006; 17: 3132-3138.
- Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009; 2009: 137072.
- Schonbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 2004; 109: II18-26.
- Schwandt A, Garcia JA, Elson P, Wyckhouse J, Finke JH, Ireland J, Triozzi P, Zhou M, Dreicer R, Rini BI. Clinical and immunomodulatory effects of celecoxib plus interferon-alpha in metastatic renal cell carcinoma patients with COX-2 tumor immunostaining. J Clin Immunol 2011; 31: 690-698.
- Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 2000; 11: 152-176.
- Grenz A. Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-alpha release. J Immunol 2012; 189: 4566-4573.
- Sadik NA, Mohamed WA, Ahmed MI. The association of receptor of advanced glycated end products and inflammatory mediators contributes to endothelial dysfunction in a prospective study of acute kidney injury patients with sepsis. Mol Cell Biochem 2012; 359: 73-81.
- Graziani G, Bordone G, Bellato V, Finazzi S, Angelini C, Badalamenti S; Gruppo di Studio Trattamenti depurativi in area critica of the Italian Society of Nephrology. Role of the kidney in plasma cytokine removal in sepsis syndrome: a pilot study. J Nephrol 2006; 19: 176-182.
- Dirkes S. Sepsis and inflammation: impact on acute kidney injury. Nephrol Nurs J 2013; 40: 125-132.
- Gomez H. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014; 41: 3-11.
- Li ZJ, Shin JM, Choi DK, Lim SK, Yoon TJ, Lee YH, Sohn KC, Im M, Lee Y, Seo YJ, Kim CD, Lee JH. Inhibitory effect of cucurbitacin B on imiquimod-induced skin inflammation. Biochem Biophys Res Commun 2015; 459: 673-678.