Research Article - Biomedical Research (2017) Volume 28, Issue 16
Prophylactic cranial irradiation in small cell lung cancer: A single-center experience
Öztun Temelli1*, Nihal Kaplan Bozdag2, Fatma Aysun Eraslan1, Hulya Gulbas1, Simay Gurocak1 and Mustafa Dikilitas2
1Faculty of Medicine, Department of Radiation Oncology, Inonu University, Malatya, Turkey
2Faculty of Medicine, Department of Medical Oncology, Inonu University, Malatya, Turkey
- *Corresponding Author:
- Öztun Temelli
Faculty of Medicine
Department of Radiation Oncology
Inonu University, Malatya
Turkey
Accepted date: July 19, 2017
Abstract
Objective: Small-Cell Lung Cancer (SCLC) constitutes about 15% of all lung cancers. It tends to frequently metastasize to the brain. Prophylactic Cranial Irradiation (PCI) is performed, when the penetration of chemotherapeutic agents to brain is insufficient. The aim of this study was to report our single-center experience with PCI in SCLC cases.
Methods: We retrospectively reviewed 28 patients with SCLC diagnosed between March 2007 and November 2016. Cranial Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) was performed from all patients to exclude metastasis before PCI. Radiation Therapy (RT) was conducted using two different instruments: a three-dimensional conformal RT-based Linear Accelerator (LINAC) instrument was used until 2013 and an Intensity-Modulated Radiation Therapy (IMRT) thereafter. All patients were treated with a total of 25-36 Gy with fraction doses of 2-2.5 Gy. Overall survival was estimated in all patients.
Results: The mean age was 56 (range: 36 to 72) y. Only one of the patients was female, while the remaining patients were all males. Twenty two patients (78.5%) were in limited stage SCLC, while six patients (21.5%) were in the extensive stage. Seventeen patients died, while 11 of them survived. The mean survival was 35 months, while it was 40 months for limited stage and 17 months for extensive stage (p=0.027). One, two, and five-year OS rates were 81.4%, 58%, and 17%, respectively. Four (14%) patients developed brain metastasis during follow-up. Of these patients, two were treated with Whole Brain RT (WBRT), one with Stereotactic RT (SBRT), and the other with best supportive care.
Conclusion: Our study results suggest that PCI is a safe, low-toxicity treatment modality used to prevent brain metastases in SCLC cases.
Keywords
Prophylactic cranial irradiation (PCI), Small cell lung cancer (SCLC)
Introduction
Small-cell Lung Cancer (SCLC) constitutes about 15% of all lung cancers [1]. SCLC is characterized by rapid doubling time, early metastasis, high sensitivity of chemotherapy and Radiation Therapy (RT) [2,3]. Only 30% of patients have presented with limited disease at the diagnosis [4]. Chemotherapy improves short-term survival. However, long-term survival outcomes are still disappointing. The median survival time is around 17 months for limited stage (LS)-SCLC patients while it is 9-10 months for extensive stage (ES)-SCLC patients [5,6].
In addition, SCLC tends to frequently metastasize to the brain. About 10% of patients present with brain metastasis at the time of diagnosis, while the cumulative risk increases to 50% or more within two years [7]. This rate increases up to 65% in autopsy series [8]. Brain metastasis is a life-threatening condition which reduces the quality of life and survival. The survival after brain metastasis is about four to five months [9]. Since the intact blood-brain barrier does not allow the passage of many cytotoxic agents, the brain is a hidden area for adjuvant systemic therapies [10]. Therefore, PCI has come to the agenda. PCI was first performed to treat children with Acute Lymphoblastic Leukaemia (ALL) in the 1970’s [11]. After a decade, it was started to perform as a part of SCLC treatment [12]. Many randomized studies have shown that PCI reduces the incidence of brain metastasis [13,14].
Methods
In the present study, we retrospectively reviewed 28 cases of SCLC diagnosed between March 2007 and November 2016. The study protocol was approved by the Inonu University Ethics Committee. The study was conducted in accordance with the principles of the Declaration of Helsinki.
The Overall Survival (OS) was calculated from the date of diagnosis until the date of death or time elapsed until January 2017. Intracranial metastasis time was calculated from the date of diagnosis. Cranial Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) was performed from all patients to exclude metastasis before PCI. All patients were treated with a total of 25-36 Gy with fraction doses of 2-2.5 Gy. Radiation therapy was conducted on two different instruments: a three-dimensional conformal RT-based Linear Accelerator (LINAC) instrument was used until 2013, and an Intensity-Modulated Radiation Therapy (IMRT) with Helical Tomotherapy.
Statistical analysis
Statistical analysis was performed by using the SPSS version 17.0 for Windows software (SPSS Inc., Chicago, IL, USA). The Kaplan-Meier method with the log-rank test was used for univariate survival analysis. Multivariate survival analysis was performed using the Cox regression analysis. A p value of <0.05 was considered statistically significant.
Results
Out of 28 patients, only one was female, whereas the remaining patients were males. The median age was 56 y (range: 36 to 72 y). All of the patients had smoking history. Twenty two patients (78.5%) had LS-SCLC, while six patients (21.5%) had ES-SCLC. The most frequent metastatic foci at the time of diagnosis of ES-SCLC patients are liver, adrenal gland and bone. Chemotherapy+thoracic RT were administered to 21 patients, while chemotherapy was administered to five patients, and two patients received postsurgical chemotherapy. In the hospital where the present study was conducted, PCI was administered to the patients with partial or complete response to chemotherapy in early or ES-and those having Eastern Cooperative Oncology Group (ECOG) performance status of two or less. Chemotherapy regimen was administered as standard treatment of cisplatin-etoposide. Twelve patients had complete response after chemotherapy whereas 16 individuals had partial response. The median interval between diagnosis and the initiation of PCI was seven months (range: 3 to 27 months). The early PCI (≤ 6 months) was performed to 13 patients, while the late PCI (>6 months) was performed to 15 patients (Table 1).
| Characteristics | n (%) |
|---|---|
| Age range (y) | |
| Mean | 56 (36-72) |
| Standart deviation | 1.63 |
| Sex | |
| Male | 27 (96.5) |
| Female | 1 (3.5) |
| Smoker | |
| Yes | 28 (100) |
| No | 0 |
| Stage | |
| Limited SCLC | 22 (78.5) |
| Extensive SCLC | 6 (21.5) |
| Site of metastasis (in ESCLC) | |
| Lung | 1 (3.5) |
| Lymph nodes | 1 (3.5) |
| Bone | 2 (7) |
| Liver | 5 (18) |
| Adrenal | 3 (10.5) |
| Treatment | |
| Chemotherapy alone | 5 (18) |
| Chemoradiotherapy | 21 (75) |
| Surgery-adjuvant chemotherapy | 2 (7) |
| Radiotherapy schedules | |
| 250 cGy × 10 fx | 18 (64) |
| 200 cGy × 18 fx | 2 (7) |
| 200 cGy × 15fx | 8 (29) |
| PCI time from diagnosis | |
| ≤ 6 months | 13 (46) |
| >6 months | 15 (54) |
| Brain metastases post PCI | |
| Limited SCLC | 3 (13) |
| Extensive SCLC | 1 (17) |
Table 1: Various characteristics of the patients.
Four patients (14%) developed brain metastasis during the follow-up programs. Two of these patients were treated with Whole Brain RT (WBRT) and Stereotactic RT (SBRT) was performed to one patient while one patient with best supportive care was used for one of them.
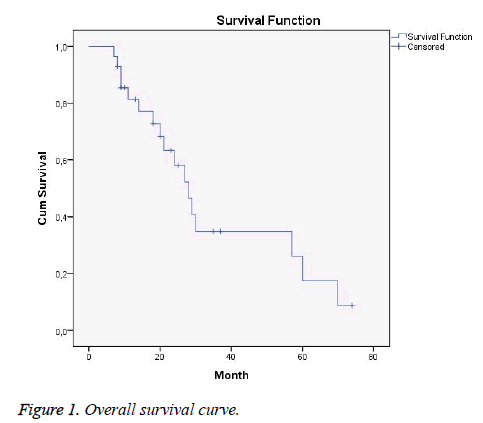
Seventeen of our patients died, while eleven of them survived. The mean survival time was 35 months, while the aforementioned time was 40 and 17 months for LS and ES, respectively (p=0.027).
One, two, and five-year OS rates were 81.4%, 58%, and 17%, respectively. According to the stage, one, two, and five-year survival rates for LS were 90%, 66%, and 22%, while these rates for ES were 50%, 33%, and 0%, respectively.
These rates were 69%, 34%, and 8%, respectively after PCI (Figure 1).
Discussion
In the present study, we report our single-center experience with PCI in SCLC cases. The mean age of patients was 56 y, consistent with the literature data, and the present study included more males than the literature [14,15]. This can be attributed to the fact that women smoke less than men in the region we conducted the study.
The survival results were better than those in the literature. In the literature, the median OS for LS-SCLC patients was about 17 months while it was about 9 to 10 months for ES-SLC patients [5,6]. In the present study, OS for LS-SCLC and ES-SCLC was calculated as 40 and 17 months, respectively. This may be due to the fact that study population sample size was small and the patients with good overall health status might receive PCI.
In LS-SCLC, several studies have shown that PCI reduces the incidence of brain metastasis [16]. Although the survival benefit of PCI has not been clear, its survival advantage has been shown in some studies. In a meta-analysis involving 987 diseases, the outcome of 7 studies was compiled, 847 (86%) of these patients were in LS-SCLC, whereas 140 (14%) of them were in ES-SCLC. The rate of development of brain metastasis after 3 y was 33.3% in the group in which PCI was performed, while it was 58.6% in the group in which PCI was not performed (p<0.001). Furthermore, 3 y survival of these two groups were 20.7% and 15.3%, respectively (p<0.001) [14]. In the ES-SCLC, PCI is recommended for patients with complete response to the chemotherapy [14]. The results are unclear for patients with partial response to the chemotherapy [15]. In the study of the European Organization for Research and Treatment of Cancer (EORTC) conducted by Slotman et al., 286 patients in ES were divided into two groups as PCI after chemotherapy and control group [17]. The symptomatic brain metastasis rate was 24 (16.8%) of 143 patients treated with PCI and 59 (41.3%) 143 patients treated without PCI (p<0.001). The median survival also increased in the RT arm.
The median survival was 6.7 months and 5.4 months for the RT arm after randomization and the control group, respectively. One-year survival rate was 27.1% (95% CI, 19.4 to 35.5) in the irradiation group and 13.3% (95% CI, 8.1 to 19.9) (p=0.003) in the control group. In the present study, PCI has been shown to reduce the incidence of brain metastasis. In addition, brain metastasis was developed in the 14% of patients, and the results were better than those in the literature [14].
The standard dose and timing of PCI are not certain. Although a scoring system ranging from 8 Gy in a fraction to 40 Gy in 20 fractions is used, the most common treatment schemes performed are those with 24-25 Gy or 30 Gy [15]. Increased doses of RT were investigated in two randomized extensive studies. Pechoux et al. compared the standard 25 Gy (10 daily fractions of 2.5 Gy) with 36 Gy (18 daily fractions of 2 Gy or 24 fractions in 16 d with two daily sessions of 1.5 Gy) [16]. The 2 y incidence of brain metastasis was detected as similar in both groups (29% vs. 23%). Moreover, 2 y OS was 42% in the group receiving standard dose RT and 37% (p=0.05) in the group receiving high-dose RT. Another study was RTOG 0212. In the aforementioned study, RT dose of 25 Gy and 36 Gy were compared. There was no significant difference between two groups in terms of either the incidence of brain metastasis at the end of the 25-month median follow-up period or overall survival [15]. In both studies, it was emphasized that the PCI dose should remain at 25 Gy. In our department, the 25 Gy RT scheme was administered as a standard.
Regarding the timing of PCI, it has been suggested that it should be applied as early as possible. In a meta-analysis, PCI administration within four to six months of induction chemotherapy initiation decreased brain metastasis development, compared to those administered PCI late (p=0.01). However, there was no difference in overall survival (p=0.39) [12]. In the present study, the PCI timing was grouped as ≤ 6 months and >6 months and there was no difference in overall survival in the development of brain metastasis (p=0.248).
Acute and chronic adverse effects may develop due to PCI. Fatigue and alopecia are the most common among acute adverse effects, while scalp erythema, headache and nausea can also be seen [18]. Neurotoxicity and intellectual disability in terms of long-term toxicity have been shown to be particularly high in older treatment modalities, in circumstances with high fraction doses or high total dose, and in cases with concomitant chemotherapy performed [18-21]. In the RTOG 0212 study, the rate of chronic neurotoxicity was found to be significantly less (60% vs. 85%, respectively, p=0.02) for patients who were administered 25 Gy compared with those receiving 36 Gy [15]. In recent years, hippocampal-sparing RT has been on the agenda to reduce the neurocognitive impairments. In the RTOG 0933 study, it was demonstrated that the memory preservation and quality of life were better with hippocampal-sparing RT technique for whole brain irradiation due to brain metastasis [22]. However, there is a need for extensive work in this area and it is necessary to wait results of on-going phase III studies (NCT01797159, NCT01780675 and NCT02635009).
In conclusion, our study results suggest that PCI is a safe, lowtoxicity treatment modality used to prevent brain metastases in SCLC cases. However, further large-scale, long-term studies are required to confirm these findings and to establish a definite conclusion.
References
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER cancer statistics review, 1975-2011. National Cancer Institute 2014
- Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of chest physicians evidence-based clinical practice guidelines. Chest 2013; 143: 400-419.
- Brade AM, Tannock IF. Scheduling of radiation and chemotherapy for limited-stage small-cell lung cancer: Repopulation as a cause of treatment failure? J Clin Oncol 2006; 24: 1020-1022.
- Hodson I, Arnold A, Zee BC, Payne D, Kostashuk EC, Evans WK, Dixon P. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer: The national cancer institute of Canada clinical trials group. J Clin Oncol 1993; 11: 336-344.
- Gaspar LE, Gay EG, Crawford J, Putnam JB, Herbst RS, Bonner JA. Limited-stage small-cell lung cancer (stages I-III): observations from the National Cancer Database. Clin Lung Cancer 2005; 6: 355.
- Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J 2010; 35: 202-215
- Komaki R, Cox JD, Whitson W. Risk of brain metastases from small-cell carcinoma of the lung related to the length of survival and prophylactic irradiation. Cancer Treat Rep 1981; 65: 811-814
- Nugent JL, Bunn PA, Matthews MJ, Ihde DC, Cohen MH, Gazdar A, Minna JD. CNS metastases in small cell bronchogenic carcinoma. Cancer 1979; 44: 1885-1893.
- Meert AP, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F, Verdebout JM, Lafitte JJ, Sculier JP. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 2001
- Zhang I, Zaorsky N, Palmer J, Mehra R, Lu B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 2015; 16: 510-521.
- Bleyer WA, Poplack DG. Prophylaxis and treatment of leukemia in the central nervous system and other sanctuaries. Semin Oncol 1985; 12: 131-148.
- Hardy J, Smith I, Cherryman G, Vincent M, Judson I, Perren T, Williams M. The value of Computed Tomographic (CT) scans surveillance in the detection and management of brain metastases in patients with small cell lung cancer. Br J Cancer 1990; 62: 684-686.
- Paumier A, Cuenca X, Le Péchoux C. Prophylactic cranial irradiation in lung cancer. Cancer Treat Rev 2011; 37: 261-265.
- Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, Aisner J. Prophylactic cranial irradiation overview collaborative group: prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med 1999; 341: 476-484.
- Le Péchoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C, Ciuleanu T, Arriagada R, Jones R, Wanders R, Lerouge D, Laplanche A. Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009; 10: 467-474.
- Le Péchoux C, Sun A, Slotman BJ, Ruysscher DD, Belderbos J, Gore E. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol 2016; 17: 277-293.
- Slotman BJ, Mauer ME, Bottomley A, Faivre-Finn C, Kramer GW, Rankin EM, Snee M, Hatton M, Postmus PE, Collette L, Senan S. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009; 27: 78.
- Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI, Choy H. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 81: 77.
- Johnson BE, Patronas N, Hayes W, Grayson J, Becker B, Gnepp D, Rowland J, Anderson A, Glatstein E, Ihde DC. Neurologic, computed cranial tomographic, and magnetic resonance imaging abnormalities in patients with small-cell lung cancer: further follow-up of 6- to 13-year survivors. J Clin Oncol 1990; 8: 48.
- Herskovic AM, Orton CG. Elective brain irradiation for small cell anaplastic lung cancer. Int J Radiat Oncol Biol Phys 1986; 12: 427.
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys 1980; 6: 1215.
- Gondi V, Pugh SL, Tome WA Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase ii multi-institutional trial. J Clin Oncol 2014; 32: 3810-3816.