Research Article - Microbiology: Current Research (2023) Volume 7, Issue 4
Profile of Hepatitis B ′e′ Antigen and Antibodies in Hepatitis B Seropositive Patients at a Tertiary Care Hospital in Mathura, Uttar Pradesh India
Anju Rani*, Shama Tomar and Bichitrananda Swain
Department of Microbiology, National Institute of Medical Sciences, Jaipur, Rajasthan, India
- Corresponding Author:
- Anju Rani
Department of Microbiology
National Institute of Medical Sciences
Jaipur, Rajasthan, India
E-mail: ananyaroy357@gmail.com
Received: 25-May-2023, Manuscript No. AAMCR-23-110949; Editor assigned: 29-May-2023, PreQC No. AAMCR-23-110949(PQ); Reviewed: 12-Jun-2023, QC No. AAMCR-23-110949; Revised: 17-Jun-2023, Manuscript No. AAMCR-23-110949(R); Published: 24-Jun-2023, DOI:10.35841/aamcr-7.4.156
Citation: Anju Rani, Shama Tomar, Bichitrananda Swain. Profile of Hepatitis B ‘e’ Antigen and Antibodies in Hepatitis B Seropositive Patients at a Tertiary Care Hospital in Mathura, Uttar Pradesh India. J Micro Curr Res. 2023; 7(4):156
Abstract
HBe Ag is a no particulate viral protein and also a marker of active replication in patients infected with Hepatitis B Virus (HBV). Hepatitis B ‘e’ antigen status is an important factor to know the prognosis and risk of infectivity in person infected with HBV. HBe Ag is an index of viral replication, infectivity, severity of disease and response to antiviral therapy. Seroconversion from HBe Ag positive to HBe Ag negative/anti HBe Ag positive phase usually heralds resolution of infection. Being an important milestone of chronic HBV infection HBe Ag status positive v/s negative is also a determinant of the mode of transmission of the virus. Development of chronic infection, following acute Hepatitis B, requires the expression of HBe Ag. Our aim was to know the seroprevalence of HBe Ag and antibody to HBe Ag (anti-HBe) in hepatitis B surface antigen (HBs Ag) seropositive individuals. 6000 individuals were screened for HBs Ag. Detection of HBe Ag and Anti-HBe antibody was done by Electrochemiluminescence immunoassay (Roche diagnostics) on COBAS e 411immunoassay analyzer. HBe Ag seroprevalence of 13.08 % indicates high infectivity among HBV-infected individuals. Present study emphasizes the need for using various serological markers for diagnosis and screening of HBV infection.
Keywords
Chronic hepatitis, Hepatitis B ‘e’ antigen, Biomarkers, ECLIA, anti-HBe antibody
Introduction
Despite immunization, Hepatitis B virus (HBV) is still prevalent Worldwide, approximately 20% of every two billion HBV infected cases continue to chronic stages, and causes nearly 900,000 deaths every year [1]. About 1 million Indians are at risk for HBV and about 100,000 die from HBV infection [2]. However, the majority of the infected population are unaware of their condition. It is also a serious threat to blood transfusion safety, especially in Endemic countries [3]. Hepatitis B virus (HBV) can cause acute to chronic liver diseases such as cirrhosis and hepatocellular carcinoma. Although HBs Ag can be detected from samples like urine, bile, tears, sweat, vaginal secretions, cerebrospinal fluid and synovial fluid but serum, saliva, and semen are reported to be infectious [4]. Several HBV biomarkers can be used for HBV diagnosis such as hepatitis B surface antigen (HBs Ag), hepatitis B surface antibody (anti-HB), hepatitis B e-antigen (HBeAg), and hepatitis core antigen (anti-HBc). The quantification of biomarkers level in the body fluids determines the infection level of HBV [5]. The hepatitis B e antigen is a product of the pre –C/C gene that has been found in hepatocytes during proliferation of hepatitis B virus and also an important diagnostic tool to determine the status of ongoing HBV infections [6,7]. Chronic HBV infection may have distinct clinical phenotypes can be divided into five phases. Phase1-High replicate, low infection state (HRLI) characterized by HBe Ag positivity, high viral load, normal or low levels of liver aminotransferases minimal necroinflammatory activity on biopsy. Phase 2- HBe Ag positive chronic hepatitis B phase with fluctuating aminotranferase levels, high HBV DNA and non-inflammatory on liver biopsy [8,9]. This phase sometimes leads to HBe Ag seroconversion. Phase 3- HBe Ag negative phase with low or undetectable levels of HBV DNA and normal aminotransferases. If this phase is prolonged, it can lead to lower rates of progression to cirrhosis and hepatocellular carcinoma (HCC) [10,11]. Phase 4- HBe Ag negative phase and represents a late immune reactive phase, with periodic fluctuating levels of aminotransferases and HBV DNA. HBV Virus may contain nucleotide substitutions in the pre-core or basal core promoter region explaining the lack of HBe Ag expression. Phase 5 is the HBs Ag negative phase where HBs Ag is lost and HBV DNA is usually undetectable in this phase, low levels of HBV DNA can albeit rarely persist in serum and the virus can be detected within the liver [8,9]. Usually HBe Ag can be detected when viral replication is high both in self- limited infections and in chronic hepatitis B; its presence for more than 10 weeks is indicative of a transition to persistant infection. 10HBe Ag can be detected in serum shortly after HBs Ag during acute HBV infections and usually disappears before HBsAg, when alanine aminotransferase (ALT) levels increases, followed by the presence of the corresponding antibody (anti-HBe). Therefore HBe Ag test is meaningful in association with the anti-HBe test for monitoring the course of HBV infection and the effect of treatment for chronic hepatitis B [11,12,13]. The Elecsys HBeAg assay uses monoclonal anti-HBe antibodies (mouse) for detection of HBeAg. Data on the prevalence of HBe Ag/anti-HBe positivity is very less, detection of HBe Ag/anti-HBe, should be done at a large scale as it is significant in the infectivity and prognosis of the HBV infection. Beside this, it is helpful to understand the frequency of highly infective HBV carriers in the given region which in turn helps to design and implement preventive and control measures such as reduction in transmission, long-term complications and death.
Materials and Methods
This Prospective study was conducted over a period of 2 years (March 2021 to March 2023) at KD Medical College, Hospital and Research Center in Mathura, India. A total of 6000 blood samples were collected after obtaining the informed consent from patients of all age groups.
Inclusion and exclusion criteria- All those patients who were seropositive for HBs Ag were included in the study. Professional blood donors, high risk group like intravenous drug abuser and patients on antiretroviral therapy were excluded in this study.
Collection and storage of samples-3-5 ml of blood was collected from each patient using strict aseptic precautions and serum was separated by using centrifuge machine. These separated samples were stored at -20?C for further investigation.
Processing of samples- All Serum samples were screened for HBs Ag by one step rapid visual assay HEPACARD manufactured by diagnostic enterprises for the qualitative detection. The method uses monoclonal antibodies immobilized on a nitrocellulose strip in thin line. The samples found to be positive for HBs Ag after repeated screening, were further tested for the presence of HBe Ag/anti-HBe. The Elycis kit was used for detection which is manufactured by Roche diagnostics. It works on the concept of Electrochemiluminescence immunoassay on the COBAS e 411 immunoassay analyzer. Total duration of assay was 18 minutes. In between two times incubation was done automatically. In 1st incubation: HBe antigen from 21 μL sample, a biotinylated monoclonal HBeAg-specific antibody, and a monoclonal HBeAg-specific antibody labeled with a ruthenium complex) form a sandwich complex. In 2nd incubation: After addition of streptavidin- coated micro particles, the complex becomes bound to the solid phase via interaction of biotin and streptavidin. The reaction mixture was aspirated into the measuring cell where the micro particles are magnetically captured onto the surface of the electrode. Unbound substances were removed with ProCell II M. Application of a voltage to the electrode then induce chemiluminescent emission which was measured by a photomultiplier. Results were determined automatically by the software by comparing the electrochemiluminescence signal obtained from the reaction product of the sample with the signal of the cutoff value previously obtained by calibration
Calculation:-The analyzer automatically calculates the cutoff based on the measurement of HBEAG Cal1 and HBEAG Cal2. The result of a sample was given either as reactive or non-reactive as well as in the form of a cutoff index (signal sample/cutoff).
Result Interpretation- COI < 1.0 Non-reactive for HBe Ag COI ≥ 1.0 Reactive for HBe Ag.
Anti-HBe antibody was also detected in serum samples by Elycys on COBAS Machine according the kit manufacturers.
Results
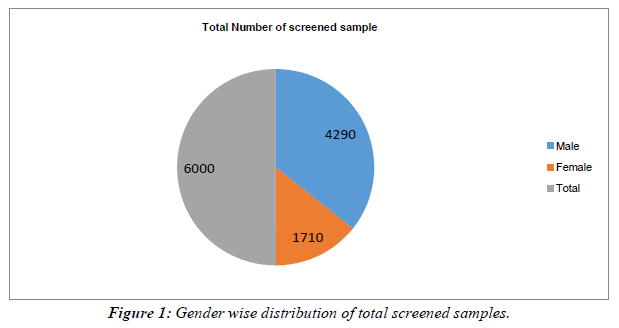
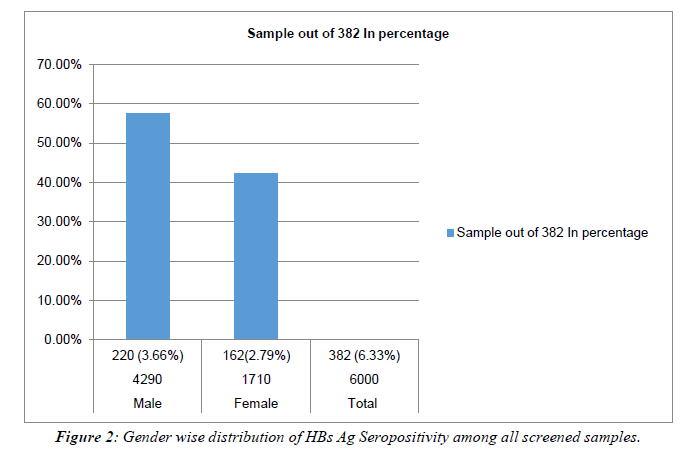
Total 6000 individuals were screened during the study period, 382 individuals were detected positive for HBs Ag with a prevalence of 6.33%. Out of these 220 were males and 162 were females with the ratio of 6:4. Table 1, Figure 1, Figure 2.
| Sex | Number of screened sample | HBs Ag positive | Percentage among positive HBs Ag |
|---|---|---|---|
| Male | 4290 | 220 (3.66%) | 57.59% |
| Female | 1710 | 162(2.79%) | 42.41% |
| Total | 6000 | 382 (6.33%) |
Table 1: Showing Gender wise distribution of HBs Ag seropositivity among all screened samples.
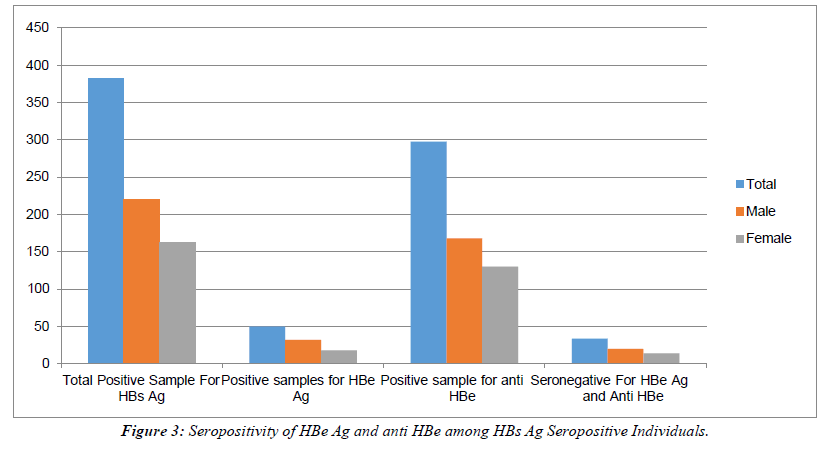
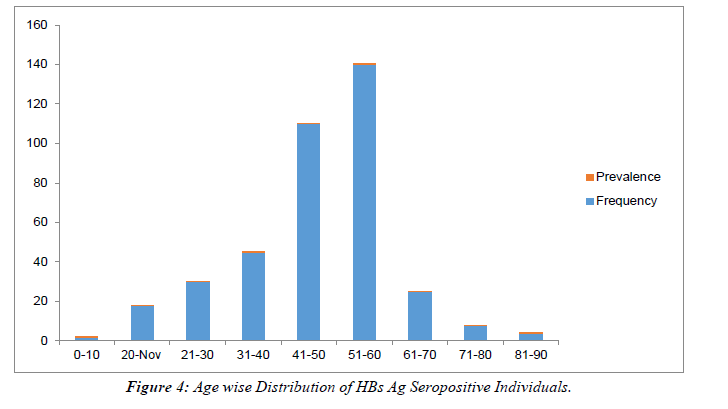
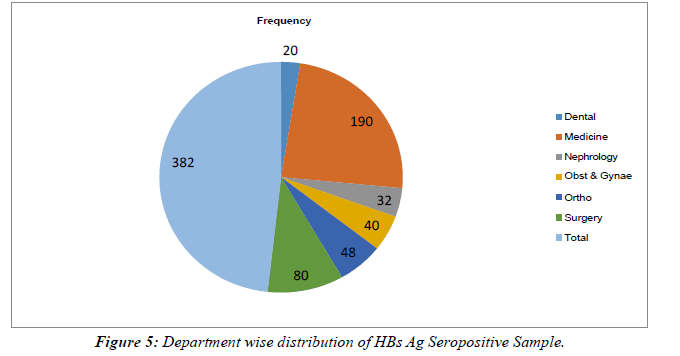
All HBs Ag-positive samples were further tested for HBe Ag and anti-HBe antibody. Total 50 samples were found positive for HBe Ag with the prevalence of 13.08% (Table 2). Out of 50 positives, 32(64%) were male and 18 (36%) were females with the ratio of 0.8:0.45. 298/382 samples were seropositive for anti-HBe antibody with a prevalence of (78%).Out of 298, 168 (56.37%) were males and 130 (43.62%) were females with a male: female ratio of 4:3. (Table 2), Figure 3. The rate of seropositivity was characterized based on age group, highest HBs Ag seroprevalence of 36.64% was found among patients with age >50 years Table 3, Figure 4. On Department wise distribution highest sample were seropositive from Medicine Department (49.7%) followed by Surgery Department (20.94%). Table 4, Figure 5.
| Total Positive Sample For HBs Ag | Positive samples for HBe Ag | Positive sample for anti HBe antibody | Seronegative For both HBe Ag and anti-HBe antibody | |
|---|---|---|---|---|
| Male | 220 | 32 (8.37%) |
168 (43.97%) | 20 (5.24%) |
| Female | 162 | 18 (4.72%) |
130 (34.03%) |
14 (3.67%) |
| Total | 382 | 50 (13.09%) |
298 (78%) |
34 (8.91%) |
Table 2: Showing Seropositivity of HBe Ag and anti HBe antibody among HBs Ag Seropositive Individuals.
| Age Group in years | Frequency | Prevalence of HBs Ag positivity |
|---|---|---|
| 0-10 | 02 | 0.5% |
| 11-20 | 18 | 4.7% |
| 21-30 | 30 | 7.85% |
| 31-40 | 45 | 11.78% |
| 41-50 | 140 | 36.64% |
| 51-60 | 110 | 28.79% |
| 61-70 | 25 | 6.54% |
| 71-80 | 08 | 2.09% |
| 81-90 | 04 | 1.04% |
Table 3: Age wise Distribution of HBs Ag Seropositive Individuals.
Showing highest infectivity of Hepatitis B Virus in age group 41-50 years with a frequency rate 140 (36.64%) followed by 51-60, 110 (28.79%) and 31-40 yrs 45(11.78%).
| Wards/Department | Frequency | Percentage |
|---|---|---|
| Dental | 20 | 5.23% |
| Medicine | 190 | 49.7% |
| Nephrology | 32 | 8.37% |
| Obst & Gynae | 40 | 10.47% |
| Ortho | 48 | 12.56% |
| Surgery | 80 | 20.94% |
| Total | 382 |
Table 4: Department wise distribution of HBs Ag Seropositive Sample
Discussion
Present study was performed to assess the seroprevalence of HBeAg/anti-HBe in HBsAg-seropositive individuals. Totally 6.33% HBs Ag seroprevalence was observed out of these 13.08% were found seropositive for HBe Ag. It may be an indication of presence of highly infective and replicative phase among HBV-infected individuals [14,15]. Some studies have shown that HBe Ag is a biomarker of active viral proliferation in hepatocytes, infectivity, and transmission and is associated with an increased risk of hepatocellular carcinoma.[16]It has been declared in some studies that HBe Ag Expression also determines whether acute HBV infection develops into a chronic infection, thus it is necessary to prerequisite that the strains of HBV infecting an individual express HBe Ag [17,18,19]. Therefore, testing for the HBe Ag can aid in identifying individuals with a high risk of developing liver cancer and in planning patient management. Several factors which are associated with an increased risk of advanced liver diseases for patients with chronic hepatitis B (CHB) have been identified. These include age, male gender, repeated episodes of severe acute exacerbation, and HBV reactivation after HBe Ag seroconversion [20, 23]. Present study declares 13.08% (50/382) positivity for HBeAg with the higher prevalence in males (64%) compared to females (36%) in the ratio of 1.7:1. HBeAg seropositivity was found to be high in patients above 50 years of age. The results are consistent with other studies [21, 22, 26]. Many physiological changes have been found associated with age, such as diminished immune response, metabolic derangements, nutritional deficiencies and greater cumulative exposure to environmental hepatotoxins may also contribute to worse outcomes of viral hepatitis in the elderly.[23, 24] In this study, anti-HBe antibody was found in 78% with higher prevalence in males and patients with age more than 40 years. Several studies with 53-90% seroprevalence of anti-HBe antibodies have been documented. HBe Ag seroconversion to anti-HBe suggests the end of active viral replication and is therefore associated with clinical resolution (self –limited) or remission (chronic disease), marking a transition from the immune active phase of the disease to the inactive carrier state [25, 26]. We observed that 8.9 % of HBsAg positive individuals were seronegative for both HBe Ag and anti-HBe antibodies. This type of situation may be seen in the early phase of seroconversion. Hepatitis B virus infection can occur without detectable HBe Ag due to infection with HBV variants containing precore stop codon mutants; while the virus can no larger produce HBeAg, disease activity is ongoing and anti- HBe may be present. In India, the majority of HBV infected persons are HBe Ag-negative, although the exact frequency and prevalence of HBeAg-negative Hepatitis has not been estimated. It would therefore be important to delineate the molecular character, viral load and response to therapy in HBe Ag-negative hepatitis B [27, 28]. In comparison with other studies, present work remains limited by the inadequacy of data in regard to the correlation of serological parameters of HBV infection with serum ALT and HBV DNA levels [29,18]. At last in this study HBV infected group was divided on the basis of several medical departments and the highest HBV prevalent rate was found from department of medicine (49.7%) followed by surgery (20.94%). Such type of distribution couldn’t be found in any other study, this may be due to its irrelevancy to the HBV infections. In view of a large population, absence of a compulsory national immunization program and increasing burden of infection and liver disease due to HBV, India may soon have the largest HBV infection pool in the world, emphasizing the relevance of its HBV epidemiology not only nationally but also internationally [31]. According to WHO’s global hepatitis strategy, the aim of elimination and eradication of HBV globally could be accomplished by reducing new hepatitis infections by 90% and deaths by 65% between 2016 and 2030, through vaccination, diagnostic tests, medicines and education campaigns [30].
Conclusion
For global eradication of HBV by 2030, it is necessary that clinicians should understand the natural history of the infection, particularly the course of spontaneous HBeAg Seroconversion. Successful elimination of HBV infection depends on efforts to make a top priority on the public-health as we are now in the second decade of this new century.
Author Contributions
Conceptualization- Dr. Shama Tomar, Dr.Bichitananda Swain
Funding
Self sourse, no another type of funds was given for the study
Acknowledgments
The authors wish to thank all participants in this study and also to thank KDMC for collecting clinical samples. It is my pleasure to acknowledge with gratitude and support of KDMC Mathura.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World journal of clinical cases. 2018 Nov 11;6(13):589.
- Premkumar M, Chawla YK. Chronic hepatitis B: challenges and successes in India. Clinical liver disease. 2021 Sep;18(3):111.
- Song Y, Bian Y, Petzold M, Ung CO. Prevalence and trend of major transfusion-transmissible infections among blood donors in Western China, 2005 through 2010. PloS one. 2014 Apr 8;9(4):e94528.
- Elgouhari HM, Abu-Rajab Tamimi TI, Carey WD. Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med. 2008 Dec 1;75(12):881-9.
- Abolfotouh MA, AlQarni AA, Al-Ghamdi SM, Salam M, Al-Assiri MH, Balkhy HH. An assessment of the level of concern among hospital-based health-care workers regarding MERS outbreaks in Saudi Arabia. BMC infectious diseases. 2017 Dec;17(1):1-0.
- Seeger C, Zoulim F, Mason WS. Hepadnaviruses. Fields virology. 2007;2:2977-3029.
- Liaw YF, Chu CM. Hepatitis B virus infection. The lancet. 2009 Feb 14;373(9663):582-92.
- Easterbrook PJ, Roberts T, Sands A, Peeling R. Diagnosis of viral hepatitis. Current Opinion in HIV and AIDS. 2017 May;12(3):302.
- McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis 2010; 14:381–396.
- Fattovich G. Natural history and prognosis of hepatitis B. InSeminars in liver disease 2003 (Vol. 23, No. 01, pp. 047-058). Copyright© 2002 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York, NY 10001, USA. Tel.:+ 1 (212) 584-4662.
- Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatology international. 2009 Sep;3:425-33.
- Rashmi KS, Misbah-Ul-Khair S, Ravikumar KL. Profile of Hepatitis B ‘e’antigen and antibodies to Hepatitis B ‘e’antigen in Hepatitis B seropositive patients at a tertiary care hospital in Bengaluru, India. International Journal of Scientific Study. 2015;3(7):53-7.
- Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis be antigen to antibody seroconversion in patients with normal serum aminotransferase levels. The American journal of medicine. 2004 Jun 15;116(12):829-34.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatol 2007; 45:507–539.
- Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A, Kennedy PT. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology. 2016 Nov 1;151(5):986-98.
- Simidele OM, Ihinacho NS, Prince NE. Prevalence of HBeAg among Hepatitis B seropositive individuals in Makurdi, Nigeria. American Journal of Biological, Chemical and Pharmaceutical Sciences. 2013;1(8):90-5.
- Forbi JC, Iperepolu OH, Zungwe T, Agwale SM. Prevalence of hepatitis B e antigen in chronic HBV carriers in North-central Nigeria. Journal of health, population, and nutrition. 2012 Dec;30(4):377.
- Chen YC, Huang SF, Chu CM, Liaw YF. Serial HBV DNA levels in patients with persistently normal transaminase over 10 years following spontaneous HBeAg seroconversion. Journal of viral hepatitis. 2012 Feb;19(2):138-46.
- Cote PJ, Korba BE, Miller RH, Jacob JR, Baldwin BH, Hornbuckle WE, Purcell RH, Tennant BC, Gerin JL. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology. 2000 Jan;31(1):190-200.
- Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002 Jun 1;35(6):1522-7.
- Hadziyannis SJ. Natural history of chronic hepatitis B in Euro-Mediterranean and African countries. Journal of hepatology. 2011 Jul 1;55(1):183-91.
- Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis be antigen to antibody seroconversion in patients with normal serum aminotransferase levels. The American journal of medicine. 2004 Jun 15;116(12):829-34.
- Rabbi FJ, Rezwan MK, Shirin T. HBeAg/anti-HBe, alanine aminotransferase and HBV DNA levels in HBsAg positive chronic carriers. Bangladesh Med Res Counc Bull. 2008 Aug 1;34(2):39-43.
- Ijoma UN, Nwokediuko SC, Onyenekwe B, Ijoma CK. Low prevalence of hepatitis Be antigen in asymptomatic adult subjects with hepatitis B virus infection in Enugu, south east Nigeria. Internet J Gastroenterol. 2010;10(1).
- Chowdhury A, Santra A, Chakravorty R, Banerji A, Pal S, Dhali GK, Datta S, Banerji S, Manna B, Chowdhury SR, Bhattacharya SK. Community-based epidemiology of hepatitis B virus infection in West Bengal, India: Prevalence of hepatitis B e antigen-negative infection and associated viral variants. Journal of gastroenterology and hepatology. 2005 Nov;20(11):1712-20.
- Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long-term outcome of hepatitis B e antigen–negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology. 2009 Jun;49(6):1859-67.
- Papatheodoridis G, Buti M, Cornberg M, Janssen HLA, Mutimer D, Pol S, et al. European Association for the Study of the Liver (EASL) clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis 2010; 14:381–396.
- Chevaliez S, Pawlotsky JM. New virological tools for screening, diagnosis and monitoring of hepatitis B and C in resource-limited settings. Journal of hepatology. 2018 Oct 1;69(4):916-26.
- Chan HL, Tsang SW, Liew CT, Tse CH, Wong ML, Ching JY, Leung NW, Tam JS, Sung JJ. Viral genotype and hepatitis B virus DNA levels are correlated with histological liver damage in HBeAg-negative chronic hepatitis B virus infection. The American journal of gastroenterology. 2002 Feb 1;97(2):406-12.
- World Health Organization.Hepatitis B factsheet. Available at:https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed February 14, 2021.
- Chowdhury A. Epidemiology of hepatitis B virus infection in India. Hepatitis B Annual. 2004 Jan 1;1(1):17.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref