Research Article - Biomedical Research (2017) Volume 28, Issue 12
Preventive effects of Malvae verticilate and Perilla frutescens var. japonica leaf on activated carbon induced constipation in ICR mice
Ruokun Yi1,2#, Na Ji2#, Xin Zhao1,2 and Kun-Young Park1,2,3*
1Chongqing Collaborative Innovation Center for Functional Food, Chongqing University of Education, Chongqing, PR China
2Department of Food Science and Nutrition, Pusan National University, Busan, South Korea
3Department of Food Science and Biotechnology, Cha University, Gyeongghi-do, South Korea
#These authors contributed equally to this work
- *Corresponding Author:
- Kun-Young Park
Chongqing Collaborative Innovation Center for Functional Food
Chongqing University of Education, PR China
Accepted on April 14, 2017
Abstract
Constipation is a common problem which ordinarily refers to as persistent, difficult, infrequent, or seemingly incomplete defecation. It includes passage of unduly dry and hard feces, sluggish action of bowel, etc. The present study evaluated the efficacy of the extract of Perilla frutescens var. japonica leaf against the activated carbon induced constipation in ICR mice. Constipation in mice was induced by the oral administration of active carbon (10% activated carbon dissolved in 0.5% arabic gum water) while the control group mice received 0.5% arabic gum water only. Constipated mice were treated with 0.5 or 1.0 g/kg body weight/day of the Perilla frutescens var. japonica leaf extract for 9 days during which all the characteristics including the feeding characteristics as body weight, dietary and drinking water amount intake, as well as fecal properties, first black stool time, gastrointestinal transit and levels of serum biomarkers such as gastrin, motilin, substance P and somatostain all were monitored thoroughly. Treatment of constipated mice shows inhibited body weight loss, dietary intake loss, with increased intestinal motility speed, fecal volume, it also shortens the first black stool time, accelerated the serum levels in gastrin, motilin, substance P, decreased the serum level somatostain which inhibited Growth Hormone (GH). Especially the anti-constipation property shown by the extract of the herb at the intake level 1.0 g/kg body weight shows the best efficacy. The effect of the extract has comparative results with biscodyl, a standard laxative drug. These findings demonstrated that Perilla frutescens var. japonica leaf can be regarded as a natural preventive method of constipation medicine.
Keywords
Perilla frutescens Var. japonica leaf, Activated carbon, Constipation, Gastrointestinal transit, Natural medicine.
Introduction
Constipation is a common problem that defined medically as fewer as three stools per week and severe constipation as less than one stool per week, it happens when the colon absorbs excessive water then the normal [1]. It is a medical condition comprising a constellation of symptoms that includes excessive straining hard stools, feeling of incomplete voidance of feces, use of digital maneuvers, or infrequent defecation. Constipation can occur without colon inflammation and is also the symptoms of several other conditions [2]. Laxatives are agents that add bulk to intestinal contents by retaining water within the bowel lumen by virtue of their osmotic effects, by that they stimulate intestinal secretion or motility thereby increasing the frequency and ease of defecation [3].
Many herbs are well known for their laxative effects that are found in a variety of dietary supplements, tea used for losing weight and colon cleaning preparation. In general use, herbs are any plant "with leaves, seeds, or flowers used for flavoring, food, and medicine. Herbs has been recognized in different system of traditional medication for the treatment of various complicated diseases and ailments, there are only few reports available on clinical use of herbs in constipation treatment that have shown promising results [4]. Recently, considerable attention has been given to the plant’s biological effects which comprise antibacterial, antiseptic, antipyretic, spasmolytic, antihistaminic and antiallergic functions in Perilla frutescens Var. japonica leaf (PL), and Perilla frutescens Var. Japonica (PF) which have been demonstrated in several studies [4]. Perilla frutescens Var. Japonica (PF) has strong qi-precipitating and phlegm-dispersing actions with the effectiveness in treating panting counterflow and phlegm cough [5]. PL and PF are also being used as food sources [6]. Malva verticilata L. (MV) as a positive control group can reduce fever and most importantly it has the function that can induce laxative effect as well as clean up the intestinal contents and there’s no necessity of further treatment. The properties such as Antiallergic, antiinflammatory, antioxidant property, neuroprotective nature and antitumor substances were demonstrated among PL, PF [7]. However, the preventive effects of PL on activated carbon induced constipation in ICR mice have not been clearly elucidated yet.
In previous research, constipation model induced by activated carbon is to demonstrate the effects of drug for constipation treatment [8,9]. A large number of cases also demonstrated that a larger dose of activated carbon can give rise to the digestive tract obstruction [10]. In this study, we tried to investigate the biological function which includes anti-constipation effects of Perilla frutescens Var. japonica leaf (PL) in vivo. Perilla frutescens Var. Japonica (PF) and Malva Verticilata L. (MV) were used as controls. All of the herb samples were powdered and extracted. Anti-constipation activity was needed to be determined in the following in vivo experiment. Firstly constipation is induced in ICR mice using activated carbon, which could not be absorbed also can be attached to the gastrointestinal surface and reduce the drainage of gastrointestinal tract either by reducing the amount of gastrointestinal fluid which are need to brought out or slowed down gastrointestinal movement or both at the same time [11]. In this paper, we use the activated carbon induced constipation model to study different concentrations of PL on constipation inhibition which is required in order to understand PL in the digestive function. Biscodyl as a positive control is a stimulating laxative drug that works directly on the colon to produce a bowl movement [12]. It is typically prescribed to get relief from constipation and for the management of neurogenic bowl dysfunction as well as to prepare a bowel part before a medical examinations [1,13,14].
To justify constipation indications in mice were change of body weight, diet uptake and drinking water amount; time changes of first black stool; Gastrointestinal (GI) transit movement situation and changes of different levels of serum biomarkers by GAS, MTL, SP and SS.
Materials and Methods
Preparations of samples
Perilla frutescens Var. japonica leaf (PL), both Perilla frutescens Var. japonica (PF), and Malva Verticilata L. (MV) as seeds were purchased from the local market (Busan, Korea). The plant material was shade dried and grinded to form powder. The samples were extracted with 70% methanol (1000 ml) for 24 h at room temperature (25.0°C) filtered through filter paper and 100 g of the PL was given to get the yield of 5.52 g. The methanol was evaporated using a rotary evaporator (Eywla, N-1100, Tokyo, Japan), concentrated substance was then dissolved in dimethylsulfoxide (DMSO; Amresco, Soion, OH, USA) to adjust it to the stock concentration (20%, w/v).
Animals
Seven-week-old female ICR mice (n=50) were purchased from Korea SLC, Inc. (Seoul, South Korea). They were sustain at the controlled temperature (temperature 25 ± 2°C, relative humidity 50 ± 5%) facility with a 12 h light/dark cycle having free access to standard rat chow diet and water. The animal protocols used in this study followed the protocols approved by the Animal Ethics Committee of Pusan National University (Busan, South Korea).
Induction of constipation in mice
In order to investigate the preventive effects of PL, PF and MV against the activated carbon induced mice constipation; the animals were divided into 10 groups of 20 mice each. The experimental design was as follows: the normal group was administered by arabic gum water for 9 days and a single dose of vehicle treatment (0.2 ml of 0.5% arabic gum, w/w); the control group received oral administration with activated carbon (0.2 ml 10% activated carbon, w/w; activated carbon dissolved in 0.5% arabic gum) in the afternoon at 6:00 pm from the sixth to ninth day. The “samples+activated carbon” groups were orally administered the dose of 0.5 or 1.0 g/kg b.w. of PL, PF and MV. The body weight, dietary intake, water intake, stools weight and stools moisture all were evaluated in the everyday morning at 9 o’clock.
Measurement defecation status of mice
These measurements were performed to determine the prokinetic action of herb samples that it is capable of propagating a prokinetic signal along the entire length of the gastrointestinal tract [15]. The excreted fecal pellets of individual mice were collected daily in the morning at 9:00 am during the whole duration of the experiment. The total number, weight, volume, and water content of each pellet were evaluated. The water content was calculated as the distinction between the wet and dry weight of pellet. After 16 h, the mice of control and sample groups received 10% activated carbon, normal group was administered 0.5% arabic gum through intragastric gavage. Then the animals were immediately placed in small transparent cages individually and allowed access to their diets and tap water ad libitum. The amount of time taken from carbon meal administration till the appearance of darkened defecation was recorded. Feces were collected, counted, water content was measured and weighted after intragastric gavage administration.
Gastrointestinal transit and defecation time
The Gastrointestinal Transit (GI) tract always releases hormones to help to regulate the digestion process [16]. On the 9th day, 10% of activated carbon was orally administered to the mice. After 1 h mark of administration, followed by the animals were humanely sacrificed and the small intestines were quickly removed. We compared the distance over which the activated carbon had travelled and the total length of the small intestine was measured. Mice were fasted for sixteen hours from the ninth day at 6:00 pm, but weren’t deprived of water. After 16 h, the mice of control and sample groups received oral administration of 10% activated carbon, the mice of normal group was administered by 0.5% arabic gum. 30 min later, mice were sacrificed by cervical dislocation under anesthesia with diethyl ether. The 10 mice of each group were dissected, and the small intestine from the pylorus to the blind intestine was carefully removed. Gastrointestinal transit of each mouse was calculated as the percentage of the distance travelled by the activated carbon meal relative to the total length of the small intestine. The equation was used to calculate GI transit (%), GI transit (%)=(distance travelled by the activated carbon)/(total length of the small intestine) × 100. Remaining mice of all groups were used administering the first black stool time after administered 10% oral administration with activated carbon.
Serum levels of gastrin (GAS), motilin (MTL), SP (substance P) and SS (somatostatin)
Serum levels of Gas, MTL, SP and SS were determined using radio-immunoassay kits (Beijing Puer Weiye Biotechnology Co. Ltd, Beijing, China). Blood from the inferior vena cava was collected into a tube and centrifuged subsequently (1,000 rpm, 20 min, 4°C). The detection range of the standard curve concentration was used for the ELISA's 15.6-1000 pg/ml according to the manufacture's protocol (Beijing Puer Weiye Biotechnology Co. Ltd. Beijing, China).
Statistical analysis
Data is presented as the mean ± SD. Differences between the mean values for individual groups were assessed with a one-way ANOVA with Duncan's multiple range test. Differences were considered significant when p<0.05. SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Results
Effect of PL, MV and PF on body weight
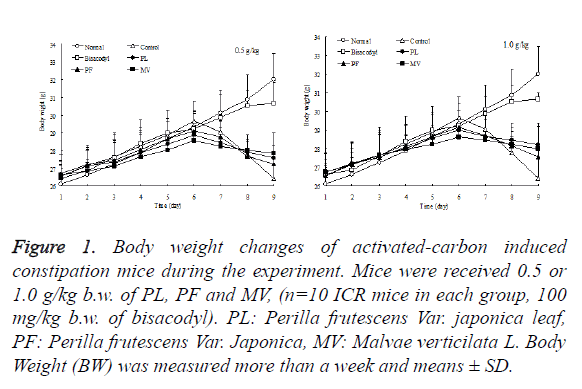
Body weight is generally considered as an important standard to measure a healthy condition of a being. Mice weight among normal, control, bisacodyl and sample groups were increased sequentially from the first to the sixth day both in low and high dose (0.5 and 1.0 g/kg b.w) (Figure 1). When they started having activated carbon from the sixth day’s afternoon, the weight of mice in control and sample groups were decreased. But weight loss of PL group mice was less than the weight loss of PF and control group which clearly demonstrated that the PL group mice was able to significantly prevent the mice weight loss induced by the activated carbon.
Figure 1. Body weight changes of activated-carbon induced constipation mice during the experiment. Mice were received 0.5 or 1.0 g/kg b.w. of PL, PF and MV, (n=10 ICR mice in each group, 100 mg/kg b.w. of bisacodyl). PL: Perilla frutescens Var. japonica leaf, PF: Perilla frutescens Var. Japonica, MV: Malvae verticilata L. Body Weight (BW) was measured more than a week and means ± SD.
Effects of PL, PF and MV on diet uptake and drinking water amount
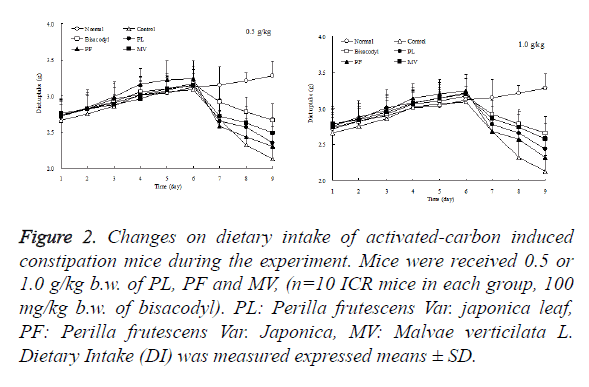
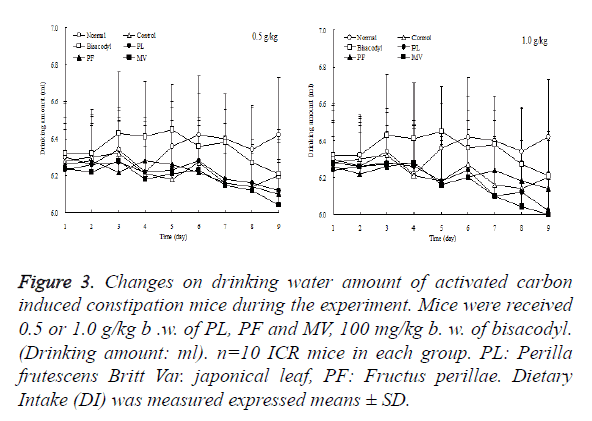
Dietary intake of mice in each group increases as time passes by and maintained from the first to sixth day. On sixth day afternoon after induction of constipation, dietary intake of control and sample groups were decreased significantly, especially control group which have least dietary intake. But PL and MV were slightly lower than PF and control group which is shown in Figure 2. Drinking water amount of each mice group was expressed in Figure 3, which showed in the whole process of the experiment occurs without obvious changes. Therefore, the effect known by the observation of the samples of constipated mice remarkably demonstrates that PL have a significant role to relieve dietary intake loss, which means it was able to relieve mice anorexia after the administration of activated carbon.
Figure 2. Changes on dietary intake of activated-carbon induced constipation mice during the experiment. Mice were received 0.5 or 1.0 g/kg b.w. of PL, PF and MV, (n=10 ICR mice in each group, 100 mg/kg b.w. of bisacodyl). PL: Perilla frutescens Var. japonica leaf, PF: Perilla frutescens Var. Japonica, MV: Malvae verticilata L. Dietary Intake (DI) was measured expressed means ± SD.
Figure 3. Changes on drinking water amount of activated carbon induced constipation mice during the experiment. Mice were received 0.5 or 1.0 g/kg b .w. of PL, PF and MV, 100 mg/kg b. w. of bisacodyl. (Drinking amount: ml). n=10 ICR mice in each group. PL: Perilla frutescens Britt Var. japonical leaf, PF: Fructus perillae. Dietary Intake (DI) was measured expressed means ± SD.
Effect of PL, PF and MV on defecation status of mice
This study divided defecation status into three parts which includes: first is defecation weight (g), which can be observed as an important expect to estimate constipation situation, if the defecation weight was heavy, that means mice had good defecation qualities; second is number of defecation pieces, which also used to justify the mice constipation situation, more defecation pieces expressed mice had good gastrointestinal movement; third is water content of defecation, that also represents mice constipation situation that means higher the water content, better is the quality of stool. From the first to the sixth day, defecation weight (g), number of defecation pieces and water content of defecation (%) in each group did not have any significant difference. Defecation weight (g), number of defecation pieces in MV group were slightly more than the other groups on the other hand water content of defecation (%) was slightly higher and PL was next to MV (Table 1). When constipation was induced, starting from the seventh day to ninth day, defecation weight (g), number of defecation pieces and water content of defecation (%) were all decreased by 0.62 g, 28 pieces and 35% respectively in control group, but on the other side defecation weight (g) was decreased by 0.4, 0.44, 0.46 and 0.4 g, number of defecation pieces was decreased by 6, 7, 6 and 7 pieces, water content of defecation (%) was decreased by 13, 11, 14 and 11% in bisacodyl and high concentration of PL, PF and MV groups, respectively (Table 2). Defecation weight (0.49, 0.52, 0.54 and 0.58 g), number of defecation pieces (12, 13, 14 and 27) and water content of defecation (15, 17, 20 and 30%) in low concentration groups were reduced more than high concentration dosage (Table 3). These results demonstrated that PL has a better defecation or curing effects after constipation, we can also state that PL can relieve constipation and has a remarkable effect to treat constipation.
| Content | Treatment | |||||
|---|---|---|---|---|---|---|
| Normal | Control | Bisacodyl | PL | PF | MV | |
| Defecation weight (g) | 0.89 ± 0.1c | 0.86 ± 0.08b | 1.05 ± 0.10a | 0.93 ± 0.0b | 0.91 ± 0.0b | 0.97 ± 0.07b |
| number of defecation pieces | 36 ± 6c | 34 ± 9c | 48 ± 4a | 39 ± 9b | 37 ± 4c | 42 ± 8b |
| Water content of defecation (%) | 47 ± 6b | 48 ± 3b | 55 ± 5a | 49 ± 5b | 48 ± 6b | 51 ± 7b |
| Each value is expressed as mean ± SD. a-cMean values with different letters in the same raw are significantly different (p<0.05) according to Duncan's multiple range test | ||||||
Table 1. The defecation status of treated mice induced by activated carbon with PL, PF and MV during the 1-6 days.
| Content | Treatment | |||||
|---|---|---|---|---|---|---|
| Normal | Control | Bisacodyl | PL | PF | MV | |
| Defecation weight (g) | 0.87 ± 0.06a | 0.24 ± 0.03e | 0.65 ± 0.10b | 0.41 ± 0.04d | 0.37 ± 0.09d | 0.48 ± 0.06b |
| number of defecation pieces | 38 ± 4b | 16 ± 6f | 42 ± 3a | 26 ± 3d | 23 ± 3e | 30 ± 2c |
| Water content of defecation (%) | 48 ± 5a | 13 ± 2f | 42 ± 5b | 32 ± 5d | 28 ± 3e | 36 ± 2c |
| Each values is expressed as mean ± SD. a-fMean values with different letters in the same raw are significantly different (p<0.05) according to Duncan's multiple range test | ||||||
Table 2. The defecation status of treated mice induced by activated carbon with low dose (0.5 g/kg) of PL, PF and MV during the 7-9 days.
| Content | Treatment | |||||
|---|---|---|---|---|---|---|
| Normal | Control | Bisacodyl | PL | PF | MV | |
| Defecation weight (g) | 0.87 ± 0.06a | 0.24 ± 0.03e | 0.65 ± 0.10b | 0.49 ± 0.04d | 0.45 ± 0.09d | 0.57 ± 0.0c |
| number of defecation pieces | 38 ± 4b | 16 ± 6e | 42 ± 3a | 32 ± 2d | 31 ± 3d | 35 ± 4c |
| Water content of defecation (%) | 48 ± 5a | 13 ± 2d | 42 ± 5b | 38 ± 4c | 34 ± 6c | 40 ± 2b |
| Each values is expressed as mean ± SD. a-eMean values with different letters in the same raw are significantly different (p<0.05) according to Duncan's multiple range test | ||||||
Table 3. The defecation status of treated mice induced by activated carbon with high dose (1.0 g/kg) of PL, PF and MV during the 7-9 days.
Effects of PL, PF and MV on first black stool defecation time in activated carbon-induced constipation mice
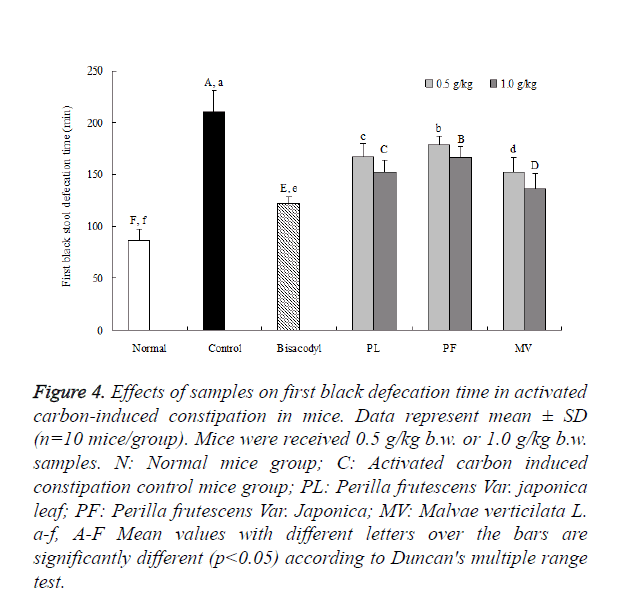
First black stool time was shown in Figure 4. Under 1 g/kg dosage concentration, first black stool defecation time were 87 ± 10, 210 ± 21, 122 ± 7 , 152 ± 12.2, 166 ± 11 and 136 ± 15.8 min in normal control, bisacodyl, PL, PF and MV groups, respectively. Under 0.5 g/kg dosage concentration, first black stool defecation in each group were significantly increased to 167 ± 13, 179 ± 8 and 152 ± 14 min in PL, PF and MV, respectively. Those results represent that biosacodly as a positive control group had a stronger effect to inhibit constipation. The time of first black stool on PL was shorter than PF. PL has a good capability to have evidential increase in gastrointestinal movement.
Figure 4. Effects of samples on first black defecation time in activated carbon-induced constipation in mice. Data represent mean ± SD (n=10 mice/group). Mice were received 0.5 g/kg b.w. or 1.0 g/kg b.w. samples. N: Normal mice group; C: Activated carbon induced constipation control mice group; PL: Perilla frutescens Var. japonica leaf; PF: Perilla frutescens Var. Japonica; MV: Malvae verticilata L. a-f, A-F Mean values with different letters over the bars are significantly different (p<0.05) according to Duncan's multiple range test.
Effects of PL, PF and MV on gastrointestinal transit in activated carbon-induced constipation in mice
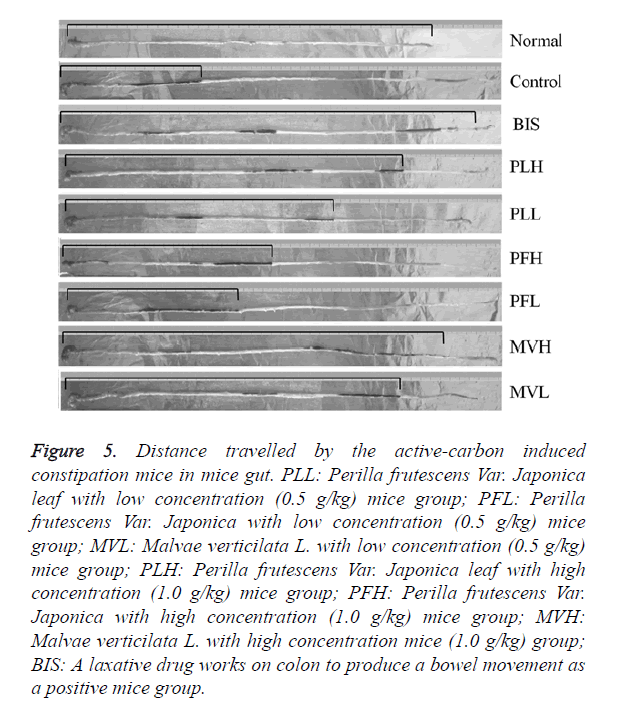
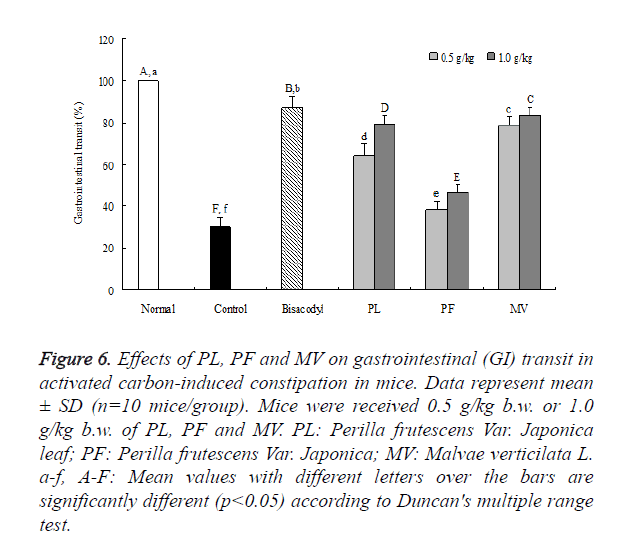
As shown in Figures 5 and 6, GI transit in each group were 97.3 ± 0.4%, 28.9 ± 4.6%, 87.3 ± 5.2%, 79.3 ± 4.1%, 46.6 ± 3.8%, 83.3 ± 4.1% in normal, control, bisacodyl, PL, PF and MV groups, respectively. The GI transits of PL, PF, MV under low dose of 0.5 g/kg b.w. were 64.2 ± 5.7%, 38.4 ± 3.9%, 78.8 ± 4.4%, which were slower than the high dose of 1.0 g/kg b.w.. The distance travelled by the activated carbon from the pylorus to the caecum was measured, and expressed as percent of the total length of the intestine. GI transits of PL in both high and low dose were more immediate than PF. These results demonstrated that the intestine situation was based on the motility of GI transit.
Figure 5. Distance travelled by the active-carbon induced constipation mice in mice gut. PLL: Perilla frutescens Var. Japonica leaf with low concentration (0.5 g/kg) mice group; PFL: Perilla frutescens Var. Japonica with low concentration (0.5 g/kg) mice group; MVL: Malvae verticilata L. with low concentration (0.5 g/kg) mice group; PLH: Perilla frutescens Var. Japonica leaf with high concentration (1.0 g/kg) mice group; PFH: Perilla frutescens Var. Japonica with high concentration (1.0 g/kg) mice group; MVH: Malvae verticilata L. with high concentration mice (1.0 g/kg) group; BIS: A laxative drug works on colon to produce a bowel movement as a positive mice group.
Figure 6. Effects of PL, PF and MV on gastrointestinal (GI) transit in activated carbon-induced constipation in mice. Data represent mean ± SD (n=10 mice/group). Mice were received 0.5 g/kg b.w. or 1.0 g/kg b.w. of PL, PF and MV. PL: Perilla frutescens Var. Japonica leaf; PF: Perilla frutescens Var. Japonica; MV: Malvae verticilata L. a-f, A-F: Mean values with different letters over the bars are significantly different (p<0.05) according to Duncan's multiple range test.
MTL, Gas, SS and SP levels in serum
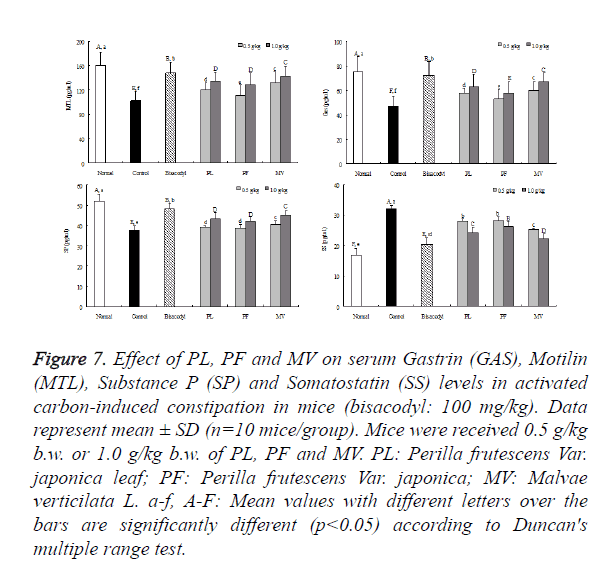
The MTL levels of normal mice were 160.3 ± 22.2 pg/ml; however, that of activated carbon-induced constipation control mice has a significant decreased that is 102.5 ± 15.5 pg/ml. Bisacodyl group as a positive control showed the level of 147.6 ± 17.8 pg/ml. Under 1.0 g/kg b.w. dose, the level of MTL in mice fed with PL and MV were 134.3 ± 14.7 and 142.6 ± 16.4 pg/ml, respectively. The levels of MTL in mice treated with 0.5 g/kg b.w. dose on PF was reduced to 128.4 ± 19.6 pg/ml, which was significantly lower level than the mice treated with both PL and MV as shown in Figure 7.
Figure 7. Effect of PL, PF and MV on serum Gastrin (GAS), Motilin (MTL), Substance P (SP) and Somatostatin (SS) levels in activated carbon-induced constipation in mice (bisacodyl: 100 mg/kg). Data represent mean ± SD (n=10 mice/group). Mice were received 0.5 g/kg b.w. or 1.0 g/kg b.w. of PL, PF and MV. PL: Perilla frutescens Var. japonica leaf; PF: Perilla frutescens Var. japonica; MV: Malvae verticilata L. a-f, A-F: Mean values with different letters over the bars are significantly different (p<0.05) according to Duncan's multiple range test.
The gas levels were shown in Figure 7. Which represent normal mice was 75.1 ± 12.3 pg/ml; whereas the activated carbon-induced constipation control mice decreased to 47.2 ± 8.2 pg/ml due to obvious reasons. The levels of Gas fed with bisacodyl mice group was 72.4 ± 11.1 pg/ml. The levels of Gas in mice treated with PL and MV were 62.8 ± 10.2 pg/ml and 67.3 ± 6.8 pg/ml, respectively, which were higher than the levels in mice treated with PF (58.1 ± 8.9 pg/ml). The similar results were also observed in 0.5 g/kg b.w. dose treated mice, the PF was decreased to 53.2 ± 7.6 pg/ml which was lower than the levels in mice with PL and MV.
Figure 7 showed the SP level in the normal group was 52.1 ± 3.4 pg/ml, whereas in the control group it was 37.7 ± 2.3 pg/ml, reflecting a marked reduction. The SP levels in both PL and MV decreased to 43.2 ± 3.2 pg/m and 45.3 ± 2.2 under 1 mg/kg b.w. dose, respectively. The level of PF was 42.1 ± 2.2 pg/ml which was slightly lower than that of the mice treated with PL and MV. The similar result of SP level in mice treated with 0.5 g/mg b.w. reflect PF decreased to 38.8 ± 1.7 pg/ml which was lower than the levels in mice treated with PL and MV.
SS levels of normal mice was 16.8 ± 2.2 pg/ml; the levels of SS in the PL and MV groups were 24.3 ± 1.78 and 22.3 ± 1.6 pg/ml, respectively, which were slightly lower than the level of the control group (32.2 ± 1.1 pg/ml). However, SS levels in the PF group were 26.2 ± 1.7 pg/ml which was higher than both PL and MV groups. These results demonstrate that PL showed lower level of SS and has a better capability of gastrointestinal movement but PL was second only to MV.
Discussion
Anorexia is an important symptom in constipation [17], so as per the observation of mice dietary intake and drinking water amount it may justify the constipation situation. Constipation refers to as infrequent bowel movements or hard to pass defecation. So, pain in the abdomen, bloating are characterized. There are many causes of constipation including medications, poor bowel habits, low fiber diet, abuse of laxatives, hormonal disorders, and disease primarily of other parts of the body that also affect the colon [18]. Therefore the defecation status is the most important criterion to judge constipation either presence or absence.
First black stool evacuated time has an impact on gastrointestinal motility. We did the observation by activated carbon to induce mice constipation in order to estimate the capability of gastrointestinal motility within mice. The shorter period of passing the stool suggests better gastrointestinal peristalsis; the longer period, indicate weaker gastrointestinal peristalsis [19]. So observed first black stool time on each mice group treated with activated carbon was able to demonstrate the preventive effect of samples on constipation.
The gastrointestinal transit was used to evaluate the constipation inhibitory effect of samples. Subsequently, 50% emptying of the small intestine takes place in 2.5 to 3 h. Finally, transit through the colon takes 30 to 40 h [20]. Gastrointestinal transit time is the time from the food taken to leave your stomach and travel through your intestine, the interval between consumption of food and it’s elimination as feces which were a handy indicator of digestive health [21]. GI transit is the effect of herb samples on the gastrointestinal motility tested on mice as per the protocol mentioned in materials and methods. The definition of constipation includes infrequent bowel movements and difficulty during defecation. Constipation most commonly occurs when the stool that forms after food is digested moves too slowly (slow transit) as its passage through the digestive tract [22]. Dehydration, changes in the diet and activity, and certain drugs are the most common causes which are need to be blame for slow transit of stool. When stool moves slowly, too much water is absorbed from the stool, and it becomes hard and dry. So, gastrointestinal transit is important standard of constipation inspection.
The serum levels of MTL, Gas and SP in patients with constipation are lower than those in healthy people, and the SS level showed higher than general people. The main function of Motilin (MTL) is to increase the migrating myoelectric complex component of gastrointestinal motility and stimulate the production of pepsin [23]. It is one of the intestinal hormones responsible for the proper filling and emptying of the gastrointestinal system in response to intake of food and hunger stimuli and responses [14]. Gastrin (Gas) is a polypeptide hormone secreted by certain cells of the pyloric gland, which strongly stimulates secretion of gastric acid and pepsin, on the other hand weakly stimulate secretion of pancreatic enzymes and gallbladder contraction. Gastrin have effects throughout the gastrointestinal tract, it can promote gastrointestinal secretion; increase in gastrointestinal movement, and at the same time it can promote the pyloric sphincter relaxation [24]. The somatostatin analogue may stimulate intestinal motor complexes and this agent has been used to treat sclerodermatous pseudo-obstruction [25]. Patients with slow transit constipation have abnormal neurotransmitters in the muscular layer of their intestinal walls [26]. These abnormalities include a deficiency of a peptide known as substance P, which is thought to contribute to peristalsis [27].
Conclusion
The present study evidentially revealed that the oral administration of the Perilla frutescens Var. japonica leaf was able to prevent constipation activity induced by activated carbon in mice. PL was able to inhibit weight loss and dietary intake loss, shorten the first black stool time, as well as improved GI transit motility, increased serum levels of MTL, GAS, SP which stimulate gastrointestinal hormone and decreased serum level SS that inhibit gastrointestinal peristalsis. These results clearly expressed that the beneficial effects of herb are many and most importantly is enhanced intestinal motility. Noteworthily, the fact is that the extract of the herb at the high dose of 1.0 g/kg body weight showed the best anti-constipation activity, which compared favorably with biscodyl and MV. These findings have lent scientific support to the use of Perilla frutescens var. japonica leaf as a functional food to prevent constipation and as a treatable medicine for the cure of the same.
Acknowledgement
This research was supported by the Construction Program of Chongqing Engineering Research Center (grant no. cstc2015yfpt_gcjsyjzx0027) and the Program for Innovation Team Building at Institutions of Higher Education in Chongqing (grant no. CXTDX201601040).
References
- Battish R, Cao GY, Lynn RB, Chakder S, Rattan S. Heme oxygenase-2 distribution in anorectum: colocalization with neuronal nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol 2000; 278: 148-155.
- El-Salhy M, Norrgard O. Colonic neuroendocrine peptide levels in patients with chronic idiopathic slow transit constipation. Ups J Med Sci 1998; 103: 223-230.
- Emmanuel AV, Tack J, Quigley EM, Talley NJ. Pharmacological management of constipation. Neurogastroenterol Motil 2009; 21: 41-54.
- Chou HJ, Kuo JT, Lin ES. Comparative antioxidant properties of water extracts from different parts of Beefsteak plant (perilla frutescens). J Food Drug Anal 2009; 17: 489-496.
- Povilaityte V, Venskutonis PR. Antioxidative activity of purple peril (Perilla frutescens L.), moldavian dragonhead (Dracocephalum moldavica L.), and roman chamomile (Anthemis nobilis L.) extracts in rapeseed oil. J Am Oil Chem Soc 2000; 77: 951-966.
- Muller-Waldeck F, Sitzmann J, Schnitzler WH, Grassmann J. Determination of toxic perilla ketone, secondary plant metabolites and antioxidative capacity in five Perilla frutescens L. varieties. Food Chem Toxicol 2010; 48: 264-270.
- Zhao G, Qin GW, Wang J, Chu WJ, Guo LH. Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt. Neurochem Int 2010; 56: 168-176.
- Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: Prepared by a task force from the American society of colon and rectal surgeons (ASCRS), the American society for gastrointestinal endoscopy (ASGE), and the society of American gastrointestinal and endoscopic surgeons (SAGES). Dis Colon Rectum 2006; 49: 792-809.
- Farrugia G, Miller SM, Rich A, Liu X, Maines MD, Rae JL, Szurszewski JH. Distribution of heme oxygenase and effects of active carbon monoxide in canine jejunum. Am J Physiol 1998; 274: 350-358.
- Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, Levitt M, Szurszewski JH. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci USA 2003; 100: 8567-8570.
- Mostafa SM, Bhandari S, Ritchie G, Gratton N, Wenstone R. Constipation and its implications in the critically ill patient. Br J Anaesth 2003; 91: 815-819.
- Wald A. Chronic constipation: advances in management. Neurogastroenterol Motil 2007; 19: 4-10.
- Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil 2001; 13: 121-131.
- Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA 2000; 97: 1851-1855.
- Feighner SD, Tan CP, McKee KK, Palyha OC, Hreniuk DL, Pong SS, Austin CP, Figueroa D, MacNeil D, Cascieri MA, Nargund R, Bakshi R, Abramovitz M, Stocco R, Kargman S, ONeill G, Van Der Ploeg LH, Evans J, Patchett AA, Smith RG, Howard AD. Receptor for motilin identified in the human gastrointestinal system. Science 1999; 284: 2184-2188.
- Becker JB, Breedlove SM. Introduction to behavioral endocrinology. Yale J Biol Med 2002; 51: 672.
- Dolk A, Broden G, Holmstrom B, Johansson C, Schultzberg M. Slow transit chronic constipation (Arbuthnot Lanes disease). An immunohistochemical study of neuropeptide-containing nerves in resected specimens from the large bowel. Int J Colorectal Dis 1990; 5: 181-187.
- Lubowski DZ, Chen FC, Kennedy ML, King DW. Results of colectomy for severe slow transit constipation. Dis Colon Rectum 1996; 39: 23-29.
- Zhao X, Suo HY, Y Qian, Li GJ, Liu ZH, Li J. Therapeutic effects of Lactobacillus casei Qian treatment in activated carbon-induced constipated mice. Mol Med Rep 2015; 12: 3191-3199.
- Goodman RD, Lewis AE, Schuck EA, Greenfield MA. Gastrointestinal transit. Am J Physiol 1952; 169: 236-241.
- Walia R, Mahajan L, Steffen R. Recent advances in chronic constipation. Curr Opin Pediatr 2009; 21: 661-666.
- Soudah HC, Hasler WL, Owyang C. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N Engl J Med 1991; 325: 1461-1467.
- Sjolund K, Ekman R, Akre F, Lindner P. Motilin in chronic idiopathic constipation. Scand J Gastroenterol 1986; 21: 914-918.
- Ryu SD, Park CS, Hwang SY, Park YC, Chung WG. Spasmogenic activity and acute toxicity of Yumijangquebo, a herbal laxative formulation. J Ethnopharmacol 2005; 101: 197-203.
- Peracchi M, Basilisco G, Tagliabue R, Terrani C, Locati A. Postprandial gut peptide plasma levels in women with idiopathic slow-transit constipation. Scand J Gastroenterol 1999; 34: 25-28.
- Silkoff P, Karmeli F, Goldin E, Ewenson A, Gilon C, Chorev M, Laufer R, Selinger Z, Rachmilewitz D. Effect of substance P on rat gastrointestinal transit. Digest Dis Sci 1988; 33: 74-77.
- Schiller LR. Review article: the therapy of constipation. Aliment Pharmacol Ther 2001; 15: 749-763.