Research Article - Biomedical Research (2017) Volume 28, Issue 4
Preventive effect of Aster tataricus on oxidative stress and biomarker of renal function in rat fed with high fat diet and sucrose diet
Xin Yao1, Xue Dong1, Hong-Shi Zhang2, Yang Wang1, Lan-Yuzhu1, Xing-Shan Liu1*1Department of Nursing, Changchun University of Chinese Medicine, Changchun, China
2Innovation Practice Center, Changchun University of Chinese Medicine, Changchun, China
- *Corresponding Author:
- Xing-Shan Liu
Department of Nursing Changchun
University of Chinese Medicine, China
Accepted on September 14, 2016
Abstract
Present investigation evaluates the antioxidant and renal protective effect of Aster tataricus (AT) obesity induced rats. Obesity was induced by High Fat Diet (HFD) and High Sucrose Diet (HSD) for the duration of 8 weeks. All the rats induced to obesity were given AT (100 and 200 mg/kg, orally (p.o.)) for next 4 weeks along with HFD and HSD. The obesity protocol resulted in a significant rise in intake of feed, body weight, body mass index and weights of adipose tissue. AT significantly decreased intake of feed, reduced body weight, adipose tissues and body mass index in a dose dependent manner. The two diets affected lipid profile and renal function, treatment of AT improved overall lipid profile in dose dependent manner and also marked a protective role on renal function by decreasing levels of creatinine and urea. AT corrected increased glucose levels and decreased Glucose 6-phosphate dehydrogenase (G6PD) activities in HFD and HSD fed rats. The treatment improved the antioxidant status by decreasing serum and renal MDA levels followed by increasing catalase activity. Decrease in Nitric Oxide (NO) was observed in HFD and HSD rats, treatment resulted in increased NO levels with increasing dose of AT. Thus present study concludes the protective effect of Aster tataricus on renal function and markers of oxidative stress in HFD and HSD fed obese rats.
Keywords
Aster tataricus, Obesity, High fat diet (HFD), High sucrose diet (HSD), Oxidative stress, Renal.
Introduction
Obesity is a condition described pathologically as an excess accumulation of body fat to the extent producing ill effects on health which leads to decreased life expectancy or life with increased health issues [1]. Rats have shown a greater amount of similarity gnomically with humans and have always been models of choice for study of obesity [2]. The protocols followed for inducing obesity in animal models comprise of strategies such as neuro-endocrine interventions, dietary or genetic changes [2].
Oxidative stress has been reported to be significantly associated with number of metabolic disorders, inflammation and also obesity [3-5]. Oxidative stress have been found to associated with damage initiated by free radicals which generate in these diseased conditions and remain non neutralized by body antioxidant defense [6]. Free radicals are unstable species causing damage to lipid containing membranes, proteins and altering DNA structures leading to cell death [7]. By-product of lipid peroxidation such as Thiobarbituric Acid Reactive Substances (TBRS) are chief oxidative stress biomarker, other such as levels of hydroperoxides and carbonyl proteins which are the markers of protein oxidation also suggest the extent of oxidative damage caused by these ROS [8,9]. Oxidative stress is also characterized by decrease in activities of body antioxidant enzymes such as Glutathione S-transferase, Glutathione Peroxidase (GPx), Superoxide Dismutase (SOD) and Catalase (CAT). These enzymes act by showing free radical scavenging activity against free radicals and prevent oxidative stress [10]. Another antioxidant enzyme namely Paraoxonase (PON1) is an important member of antioxidant family which is reported to be associated with high-density lipoproteins. The esterase enzyme is believed to detoxify lipid peroxides, PON1 is distributed in tissues such as liver, heart, brain, kidneys and lungs [11]. Obesity has been reported to be associated with production of oxidative stress [12]. Obesity exerts damaging effects on organs in the body such as liver, heart and kidney with fatty liver and diabetic nephropathy being the most possible outcomes of obesity [13].
Number of experiments has been reported indicating relation of Cardiovascular System (CVS) with hepatic system in obesity, however there is a shortfall of biochemical data indicating effect of obesity on pathophysiology of renal system. Also the exact pathogenesis of diabetic nephropathy is still under scientific scanner due to the lack of suitable animal models.
Aster tataricus (AT) is one of the commonly used herbs in Chinese medicine from past 2000 years. The root chiefly contains triterpenes and triterpene saponins; the herb has been discovered to show activity on bronchial system, anti-infective, antibacterial, antifungal, antitussive, expectorant and stimulant [14-17].
The aim of the present research work was to build up an animal model for obesity using High Fat Diet (HFD), High Sucrose Diet (HSD) and to study effects of methanolic extract of AT on changes in levels of oxidative stress markers and biomarkers of kidney function. The research would predict the possible mechanisms which could correlate obese condition with improper renal function followed by treatment with Aster tataricus.
Materials and Methods
Extraction of plant material
Roots of Aster tataricus were procured from local supplier and authentified from Institute of Medicinal Plant Development, Beijing. Extraction of Aster tataricus (AT) roots was done by maceration. Dried roots were powdered and kept it with 70% ethanol for 72 hrs. Thereafter from the extract ethanol was evaporated at low temperature by rota vapour apparatus. The dried extract was stored in a desiccators and 6.3 % w/w yield was obtained.
Animals, diet plan and treatments
Diet plan: For the study three types of diet plans were opted. The control (group-1) was fed with normal rat chow diet, group-2 was given HFD only (35%) and group-3 was also fed with only HSD (65%) in order to induce the rats with obesity. The preparation procedure was as documented [2,3], the composition of diets was as follows.
1. Rat chow diet (Normal): Briefly the diet consisted of 350 g of concentrate, 600 g of corn, sodium chloride, dicalcium phosphate, magnesium oxide, calcium carbonate and vitamins. The normal standard diet constituted starch (60%), protein (20%), sucrose (5%), Fat (5%), minerals and vitamins (5%), fibers (5%).
2. High fat diet (HFD): The diet consisted of 300 g concentrates, 300 g beef tallow, 350 g corn, vitamins, minerals and fibers comprising total 50 g according to Kim et al. [2] and. The diet constituted protein (20%), starch (35%), fat (35%), sucrose, vitamins, minerals (5%) and fibres.
3. High sucrose diet (HSD): Consisted of sucrose 600 g, methionine 100 g, concentrate 250 g, methionine 100 g, fibers 50 g, vitamins and minerals. The chemically the diet composed of 65% sucrose (65%), protein (20%), starch (5%), 5% each fat and fibres, vitamins and minerals as described.
Experimental animals
Present study was approved and was as per guidelines given by the Committee of Scientific Ethics at Beni Suef University.
For the research work male Wister rats ranging in body weight from 100-130 g ageing 8 wks. Rats were purchased from Jiangsu University and were occupied in metal cages individually maintained at 37°C and 12 hrs. light dark cycle. Rats were accessed to water and special diet in free manner, during the course weights of animals were recorded every week, food intake was monitored daily.
Acute toxicity and treatment regimen
Acute toxicity for ethanolic root extract of Aster tataricus was performed as per the Organisation for Economic Co-operation and Development (OECD) guideline (423). In this study overnight fasted male wistar rats were used for the dosing of AT. Lethal dose of AT was estimated and 1/10th and 1/20th of LD50 considered as effective dose. AT extract was weighed and was diluted in a mixture of 2% tween-20 in water the solution was mixed uniformly the mixture was administered orally daily. Control rats were administered 2% tween-20 in water.
Experimental
Experiment protocol and animal grouping
The experiment continued for time period of 12 week and comprised of two phases (1. The induction phase and 2. The treatment phase) with number of rats 48.
1. The induction phase: The phase consisted of protocol to induce obesity which began from 1st week to the 8th week involved with feeding of HFD or HSD. Experimental rats were distinguished into 3 groups named control group, HFD group and the HSD group.
The rats of control group (n=8) were fed with standard rat chow diet described earlier throughout the experimental protocol i.e. for 12 weeks. Animals of group on HFD (n=24) were maintained on High Fat Diet (HFD) prepared as discussed in diet plan. HSD group rats (n=24) were given high sucrose diet throughout the experiment.
2. The treatment phase: The phase began from 8th to 12th week, each of the group i.e. HFD and HSD which was further segregated into three groups under each treatment. The HFD animals (n=8) received high fat diet with no any treatment, HFD rats with AT treatment (100 mg/kg) (n=8) (HFD+AT 100 mg), HFD rats with AT (200 mg/kg) (HFD+AT 200 mg). Similarly rats of HSD group were divided into rats maintained on high sucrose diet without treatment (n=8), Rats (n=8) receiving high sucrose diet along with AT 100 mg/kg (HSD +AT 100 mg) (8 rats) and HSD treated rats receiving AT 200 mg/kg (HSD+AT 200 mg).
Average food consumed by each experimental rat was calculated by deducing the quantity of food provided from the leftover food daily [6]. The corresponding amount of energy intake for diet was obtained by multiplying consumed diet with factors of respective diets i.e. for rat chow diet (standard), HFD rats and HSD rats were 2.813, 5.13 and 3.41 respectively [6]. The Body Mass Index (BMI) and weight of animals was evaluated every week.
Samples preparation (Blood and tissue)
Samples of blood were collected in centrifuge tubes employing retro orbital plexus method in rats using glass capillary tubes of uniform diameter in fasting condition. The blood samples at room temperature were allowed to coagulate and were centrifuged at 5000 rpm for 10 minutes to obtain serum. For biochemical measurements supernatants of serum were collected by clean and dry syringes and were stored at -20°C. Simultaneously another blood samples for estimation of glucose were added up with sodium fluoride as anticoagulant.
For studies of biochemical parameters in tissues the sampling was done at the end of all experimental protocols. For the same rats were sacrificed by process of decapitation. In the process further abdominal incision was made for separating kidney and adipose tissues of perirenal region, mesenteric region and the epididymal region. The obtained kidney was rinsed by saline solution and weighed after drying and then subjecting to homogenization, the extract of homogenate was subjected to biochemical analysis of Lipid per oxidation (MDA), Catalase (CAT) and Nitrous Oxide (NO).
Biochemical markers in serum and tissue
Levels of plasma glucose were analysed using Stanbio Kits (USA) [7]. Serum urea and serum creatinine were quantified by colorimetric method using kits obtained from Diamonds Diagnostics [8,9]. Total lipid profile i.e. Total Cholesterol (TC), Triglyceride (TG), Low Density Lipoproteins (LDL) and High Density Lipoproteins (HDL) along with activity of G6PD were measured in serum [10] calorimetrically using kits procured from Biodiagnostic Egypt. The levels of MDA in kidney tissue and serum were estimated by allowing the MDA to react with TBA to yield a red colored product measured by spectrophotometer (532 nm) [11]. The Catalase Activity (CAT) of renal tissue was quantified in accordance to process elaborated by Cohen et al. [12]. The Nitrous Oxide (NO) levels in both renal and serum samples was measured as per described by Miranda et al. [13].
Statistical analysis
All the data were reported as mean ± S.E.M and analysed using one-way Analysis of Variance (ANOVA). Value of p<0.05 was considered statistically significant. All the data was analysed using Graph Pad Prism 5 software (San Diego, CA, USA).
Results
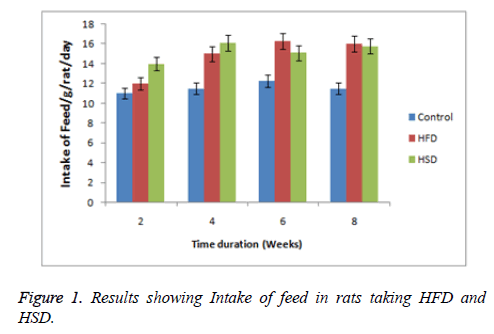
Effect on intake of feed, BW and BMI
In the present research work feed intake (Figure 1 and Table 1), BW and BMI were found to increase significantly throughout the experiment. Intake of HFD, HSD significantly decreased intake of food, BW and BMI (Figure 1, Tables 2 and 3).
| Intake of feed/gm/rat/Day | ||||||||
|---|---|---|---|---|---|---|---|---|
| HFD | HSD | |||||||
| Week 9 | Week 10 | Week 11 | Week 12 | Week 9 | Week 10 | Week 11 | Week 12 | |
| Control | 11.9 ± 0.8a | 12 ± 0.7b | 12.6 ± 0.4c | 14.1 ± 0.2b | 10.9 ± 0.6 | 11.8 ± 0.9 | 12.2 ± 0.6 | 13.4 ± 0.7 |
| Negative | 13.5 ± 0.7a | 14.6 ± 0.8b | 15.1 ± 0.9c | 15.6 ± 0.4bd | 14.2 ± 0.8 | 15 ± 0.7 | 15.5 ± 0.7 | 14.9 ± 0.6 |
| AT(100mg) | 13.9 ± 1.5a | 13.3 ± 1.1b | 12.8 ± 0.7c | 11.9 ± 0.6d | 13.9 ± 0.9 | 13.1 ± 0.9 | 12.8 ± 0.5 | 11.7 ± 0.8 |
| AT(200mg) | 12.9 ± 1.5a | 12.1 ± 1.1b | 11.2 ± 0.7c | 10.6 ± 0.6d | 13.2 ± 0.7 | 12.5 ± 0.8 | 11.9 ± 0.9 | 10.2 ± 0.6 |
Results are mean ±SD. Values having same alphabets superscripted indicate non-significant results while different alphabets represent significant variations (p<0.05).
Table 1. Results of HFD and HSD followed by treatment of AT (100 mg/kg and 200 mg/kg) on intake of feed in rats. Intake
| Body Weight (BW) (gm) | ||||||
|---|---|---|---|---|---|---|
| HFD | HSD | |||||
| Initial | Week 8 | Week 12 | Initial | Week 8 | Week 12 | |
| Control | 122 ± 5.9 | 132 ± 7.8 | 148.5 ± 6.4 | 125 ± 7.2 | 136 ± 6.5 | 151 ± 4.8 |
| Negative control | 121 ± 7.1 | 225 ± 8.9 | 312.1 ± 7.1 | 125 ± 8.1 | 201 ± 7.5 | 285 ± 9.8 |
| AT(100mg/kg) | NT | 201 ± 10.5 | 235.2 ± 9.2 | NT | 188 ± 6.9 | 210 ± 7.8 |
| AT(200mg/kg) | NT | 185 ± 9.6 | 165.2 ± 9.8 | NT | 172 ± 8.2 | 192 ± 7.3 |
Results are mean ±SD. Values having same alphabets superscripted indicate non-significant results while different alphabets represent significant variations (p<0.01).
Table 2. Results of HFD and HSD followed by treatment of AT (100 mg/kg and 200 mg/kg) on Body Weight (BW).
| Body mass index (BMI) g/cm2 | ||||||
|---|---|---|---|---|---|---|
| HFD | HSD | |||||
| Initial | Week 8 | Week 12 | Initial | Week 8 | Week 12 | |
| Control | 8.2 ± 0.9 | 8.4 ± 0.9 | 11.9 ± 0.9 | 8.3 ± 0.7 | 8.4 ± 0.8 | 11.3 ± 0.7 |
| Negative control | 9.8 ± 1.1 | 13.9 ± 1.1 | 16.7 ± 0.9 | 9.6 ± 0.3 | 12.8 ± 0.3 | 15.5 ± 0.5 |
| AT(100mg/kg) | NT | 12.8± 0.5 | 14.1 ± 0.1 | NT | 12.3 ± 0.7 | 13.7 ± 0.6 |
| AT(200mg/kg) | NT | 12.1 ± 0.6 | 11.2 ± 0.3 | NT | 11.9 ± 0.6 | 12.5 ± 0.8 |
Results are mean ±SD. Values having same alphabets superscripted indicate non-significant results while different alphabets represent significant variations (p<0.05).
Table 3. Results of HFD and HSD followed by treatment of AT (100 mg/kg and 200 mg/kg) on Body Mass Index (BMI).
Effect on serum lipid profile and renal function
Intake of two diet plans HFD and HSD resulted in significant increased levels of serum urea, creatinine and altered lipid profile compared to control rats. The levels of urea, creatinine, TC, TG and LDL were on significantly higher side in HFD rats compared to HSD fed rats (p<0.01). Treatment of AT in both HFD and HSD fed caused significantly decreased levels of serum urea and creatinine, TG, TC and LDL in comparison to non-treated rats fed with high fat and high sucrose (Table 4).
| HFD | HSD | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Negativecontrol | AT (100 mg) | AT (200 mg) | Control | Negativecontrol | AT (100 mg) | AT (200 mg) | |
| TC (mg/dl) | 78.5 ± 2.8a | 321 ± 7.8b | 150.5 ± 4.5 | 125 ± 6.4c | 77.5 ± 3.5 | 314 ± 9.8 | 175 ± 1.5 | 169 ± 3.2 |
| TG (mg/dl) | 72.5 ± 2.7a | 352 ± 9.8b | 275.5 ± 7.5 | 226 ± 7.9c | 73.5 ± 2.5 | 344 ± 9.6 | 219 ± 7.6 | 206 ± 9.6 |
| LDL (mg/dl) | 29 ± 1.5a | 273.5 ± 11.5b | 132.5 ± 12.5 | 74.5 ± 6.9c | 30.2 ± 1.9 | 220 ± 7.6 | 88 ± 1.2 | 69 ± 2.1 |
| HDL mg/dl) | 48.6 ± 1.9a | 69.5 ± 4.6a | 68.4 ± 3.5 | 67.5 ± 2.3a | 47.6 ± 2.1 | 74 ± 2.3 | 69 ± 1.3 | 65 ± 1.5 |
| Creatinine (mg/dl) | 0.26 ± 0.01a | 0.76 ± 0.02b | 0.54 ± 0.05 | 0.36 ± 0.02ac | 0.24 ± 0.02 | 0.79 ± 0.03 | 0.35 ± 0.01 | 0.3 ± 0.02 |
| Urea (mg/dl) | 16.5 ± 1.1a | 34.2 ± 1.9b | 20.1 ± 1.2 | 16.8 ± 1.3ac | 16.3 ± 1.2 | 31 ± 1.1 | 17.5 ± 1.4 | 15.2 ± 0.5 |
Results are mean ±SD. Values having same alphabets superscripted indicate non-significant results while different alphabets represent significant variations (p<0.05).
Table 4. Results of Lipid profile and kidney function followed by treatments of HFD and HSD followed by AT in rats.
Effect on levels of glucose (plasma), activity of G6PD and the adipose tissues
HFD diet resulted in increased glucose levels against control while the treatment of AT in HFD+AT treated group resulted in lowering of glucose levels (p<0.01) (Table 5). The activity of G6PD decreased significantly in rats fed with HFD whereas the activity levels were higher in rats of HSD and HSD+AT treated (p<0.01) versus the control. Evaluation of adipose tissue suggested a significant increase in mesenteric tissues the perirenal tissues and the epididymal tissues in both HFD and HSD against control rats. AT treatment resulted overall decrease in weight of all the three adipose tissues compared to the untreated HFD and HSD group of rats. The treatment of AT in HFD and HSD fed rats caused 34, 60, 49 % and 27, 27, 37% reduction respectively of mesenteric, perirenal and epididymal tissues against the untreated HFD and HSD fed rats.
| HFD | HSD | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Negativecontrol | AT (100 mg) | AT (200 mg) | Control | Negativecontrol | AT (100 mg) | AT (200 mg) | |
| Glucose (mg/dl) | 52.6 ± 1.8a | 116 ± 4.7b | 105 ± 1.5 | 96.4 ± 7.2c | 55.1 ± 1.5 | 86.4 ± 6.7ad | 68 ± 1.1 | 57.6 ± 4.2ad |
| G6pD (mU/ml) | 0.29 ± 0.01a | 0.18 ± 0.01b | 0.16 ± 0.01 | 0.15 ± 0.01b | 0.28 ± 0.01 | 0.46 ± 0.02c | 0.55 ± 0.1 | 0.49 ± 0.02c |
| Mesenteric fat (g) | 1.9 ± 0.17a | 3.2 ± 0.44b | 2.7 ± 0.5 | 2.1 ± 0.29c | 1.8 ± 0.11 | 2.9 ± 0.26c | 2.4 ± 0.1 | 2.1 ± 0.22e |
| Perirenal fat (g) | 2.6 ± 0.33a | 3.5 ± 0.25b | 2.1 ± 0.15 | 1.4 ± 0.22ac | 2.7 ± 0.3 | 3.3 ± 0.41bc | 2.9 ± 0.3 | 2.4 ± 0.21d |
| Epididymal fat (g) | 0.95 ± 0.05a | 2.55 ± 0. 6b | 0.7 ± 0.05 | 1.3 ± 0.33c | 0.9 ± 0.01 | 2.1 ± 0. 4b | 1.7 ± 0.5 | 1.32 ± 0.15dc |
Results are mean ±SD. Values having same alphabets superscripted indicate non-significant results while different alphabets represent significant variations (p<0.05).
Table 5. Results of blood glucose, G6pD and adipose tissues followed by treatments of HFD, HSD and AT in rats.
Effects on oxidative stress markers (MDA, activity of catalase and levels of NO)
Rats fed with high fat and sucrose diets resulted in marked elevation of serum MDA compared to control rats. The rats receiving HFD and HSD diet and treatment of AT demonstrated deceased serum MDA levels compared to respective untreated rats. The intake of both the diets i.e. High fat and sucrose affected kidney tissue levels of MDA and activity of catalase, the levels of MDA were high followed by decreased catalase activity. Treatment of AT significantly improved the antioxidant status by decreased MDA levels and elevated catalase activity compared to non AT treated rats (Table 6).
| HFD | HSD | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Negativecontrol | AT (100 mg) | AT (200 mg) | Control | Negativecontrol | AT (100 mg) | AT (200 mg) | |
| Serum MDA (nmol/ml) | 2.4 ± 0.30a | 4.6 ± 0.33b | 3.1 ± 0.2 | 2.5 ± 0.44a | 2.5 ± 0.1 | 6.9 ± 0.40c | 5.1 ± 0.1 | 4.3 ± 0.40b |
| Renal MDA (nmol/ml) | 1.3 ± 0.13a | 6.9 ± 0.22b | 4.5 ± 0.4 | 3.9 ± 0.44b | 1.3 ± 0.2 | 5.1 ± 0.6c | 3.9 ± 0.4 | 2.85 ± 0.2c |
| Renal catalase (U/gm) | 9.8 ± 0.30a | 4.5 ± 0.44b | 6.8 ± 0.7 | 8.2 ± 0.45ac | 9.9 ± 0.2 | 4.7 ± 0.80b | 6.4 ± 0.2 | 8.6 ± 0.50ac |
| Serum NO (µmol/L) | 2.9 ± 0.23a | 2.5 ± 0.30b | 3.2 ± 0.1 | 4.1 ± 0.30ac | 2.7 ± 0.1 | 1.9 ± 0.10d | 3.1 ± 0.8 | 5.9 ± 0.40e |
| Renal NO (µmol/L) | 1.5 ± 0.02a | 0.85 ± 0.03b | 0.95 ± 0.05 | 1.1 ± 0.01c | 1.4 ± 0.1 | 0.75 ± 0.04b | 0.88 ± 0.01 | 1.1 ± 0.02c |
Results are mean ±SD. Values having same alphabets superscripted indicate non-significant results while different alphabets represent significant variations (p<0.05).
Table 6. Results of antioxidant status (renal and serum) followed by treatments of HFD, HSD and AT in rats.
The two diets (HFD and HSD) resulted in significantly decreased NO levels in both serum and renal tissues compared to control. The treatment of AT however attenuated the levels showing a significant rise of NO in serum and renal tissues of the rat fed on HFD and HSD (Table 6).
Discussion
Obesity is one of the major risk factors leading to diabetes [18], fatty liver [19], cardiac diseases such as congestive heart failure and coronary heart disease [20] beside this obesity also effects and leads to renal failure. Feeding animals such as rats with high fat diet have proved to be an effective model to predict the effects of dietary fat in humans [21]. Rats have hence proved to be useful models for induction of obesity as they gain the weight readily on feeding them with HFD [2].
Present investigation evaluates the effect of Aster tataricus on obese rats (fed with HFD and HSD) and was evaluated for amount of food intake, body weight, BMI, kidney function and extent of oxidative stress involved. The present study shows effects of HFD and HSD on the biomarkers of kidney and oxidative stress and there modulation by treatment of AT. The study also proves beneficial role of AT in management of obesity induced renal dysfunction.
High fat diet and high sucrose diet have been documented to induce renal disturbances causing inflammation and enlargement of connective tissues, beside renal function HFD and HSD also lead to increased levels of oxidative stress [22]. Together both oxidative stress and inflammations are important pathogenic factors for cellular death and can cause renal damage.
Both high fat and sucrose diets ensued significant increase in intake of diet with increasing time in rats. Overconsumption of fat has been found to be outcome of factors such as palatability and higher density of energy, while increased intake of HSD has been contributed to sucrose affecting appetite centers in brain via insulin and leptin pathways. Both the diets also caused increase in total body weight and BMI which possibly be due to high calorie diet causing deposition of adipose tissues and inducing mitochondrial dysfunction into them [23,24].
Treatment of AT caused significant downfall in intake of feed (both HFD and HSD) in a dose dependent manner causing a significant downfall in BW and BMI compared to control group (Tables 2 and 3).
Rats fed on high fat and sucrose diets showed makeable elevation in levels of serum TC, TG and LDL against control rats (Table 4). The results may be correlated to presence of beef tallow in HFD causing hypercholesterolemia [25] and presence of sucrose in HSD resulting in hypertriglyceridemia [26].
Treatment of AT resulted in improved lipid profile with increasing dose in both HFD and HSD fed rats. Results evidenced decreased serum levels of TC, TG and LDL in both groups. The lipid profile improved with treatment time especially in the 12th week of study.
Both the diets comprising fats and sucrose respectively altered renal activity, biomarkers of kidney function such as serum creatinine and urea levels were on significantly upper side. Induction of treatment with AT caused improvement in renal function biomarkers marked by decreased serum levels of creatinine and urea compared to corresponding controls and the non AT treated rats (Table 4).
Results of serum glucose suggested occurrence of obesity induced type-2 diabetes in HFD rats with significantly elevated levels compared to controls , HSD do not produced elevated glucose levels showing results in accordance to study reported by Fukuchis et al. [27]. Treatment of AT significantly improved serum levels of glucose in HFD rats compared to respective non treated rats. The results of hyperglycaemia in HFD rats established correlation between results of renal function and fat induced obesity. AT clearly demonstrated its role in possible therapy in management of type-2 diabetes by declining the resistance of insulin. The possible mechanism may be via increasing oxidation of glucose and improving the action of insulin.
The activities of G6PD decreased significantly in HFD rats, the outcomes may be due to increased glucose levels associated with HFD leading to phosphorylation and inhibition of G6PD activity causing decrease in NADPH and increased oxidative stress [28]. Treatment of AT showed significant increase in G6pD activities in dose dependent manner in treated rats compared control group rats (Table 5).
Results of our study showed significant deposition in adipose tissues of mesenteric, perirenal and epididymal region in both i.e. high fat and sucrose diet rats (Table 5). Results already have evidenced potentiating role of HFD and HSD in increasing the BW. Treatment of AT in both diet group demonstrated decreased depositions of adipose tissues compared to control. The activity of AT may be contributed to causing decreased intake of diet and BW.
Evaluation of parameters of oxidative stress in HFD and HSD fed rats suggested significantly decreased renal catalase activity and increased levels of MDA compared to control in both the diet groups suggesting increase in production of ROS in renal tissues (Table 6). The decreased renal catalase activity evidence a direct relation between obesity induced oxidative stress whereas and increased renal MDA levels may be result of altered lipid profile due to raised LDL, TC and TG causing peroxidation of lipids. Treatment of AT improved activity of catalase with increased dose in kidney and decreased MDA levels establishing a correlation between correcting action of AT on lipid profile. Similarly AT also reduced MDA levels in serum. Results of renal and serum NO levels suggested significant decrease in levels of NO in HFD and HSD fed rats compared to control. Treatments of AT resulted in increased levels of NO in both HFD and HSD rats.
Conclusion
From the study we conclude that intake of high fat and sucrose diet are the best available techniques to induce obesity and study associated biomarkers such as serum urea, creatinine, lipid profile, glucose and markers of oxidative stress such as catalase activity, MDA and NO. Study confirmed obesity associated with altered lipid profile, renal function and increased ROS production. The study evidenced ameliorating role of methanolic extract of Aster tataricus against obesity induced renal function parameters such as urea and creatinine. Study establishes a novel correcting role of AT in obesity induced type-2 diabetes in improving overall lipid and antioxidant profile in rat models.
References
- Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197-1209.
- Von Diemen V, Trindade EN, Trindade MR. Experimental model to induce obesity in rats. Acta Cir Bras 2006; 21: 425-429.
- Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K. Evidence for contribution of vascular NAD (P) H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free RadicBiol Med 2004; 37: 115-123.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752-1761.
- Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent ProgHorm Res 2004; 59: 207-223.
- Valdecantos MP, Perez-Matute P, Martinez JA. Obesity and oxidative stress: role of antioxidant supplementation. Rev Invest Clin 2009; 61: 127-139.
- Mishra KP. Cell membrane oxidative damage induced by gamma-radiation and apoptotic sensitivity. J Environ PatholToxicolOncol 2004; 23: 61-66.
- Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J ObesRelatMetabDisord 2002; 26: 1159-1164.
- Uzun H, Konukoglu D, Gelisgen R, Zengin K, Taskin M. Plasma protein carbonyl and thiol stress before and after laparoscopic gastric banding in morbidly obese patients. ObesSurg 2007; 17: 1367-1373.
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann Bot 2003; 91: 179-194.
- Senti M, Tomas M, Fito M, Weinbrenner T, Covas MI. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J ClinEndocrinolMetab 2003; 88: 5422-5426.
- Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006; 30: 400-418.
- Pi-Sunyer FX. The obesity epidemic: Pathophysiology and consequences of obesity. Obes Res 2002; 10: 97S-104S.
- Bown D. Encyclopaedia of herbs and their uses. Dorling Kindersley London 1995.
- Medicinal plants in the Republic of Korea World Health Organisation, Manila 1998.
- Yeung HC. Handbook of Chinese herbs and formulas. Inst Chinese Med Los Angeles 1985.
- Duke JA, Ayensu ES. Medicinal plants of China Ref Publ 1985.
- Pagotto U, Vanuzzo D, Vicennati V, Pasquali R. Pharmacological therapy of obesity. G ItalCardiol (Rome) 2008; 9: 83S-93S.
- Marovia‡ D. Elevated body mass index and fatty liver. SrpArhCelokLek 2008; 136: 122-125.
- Artham SM, Lavie CJ, Milani RV, Ventura HO. The obesity paradox: Impact of obesity on the prevalence and prognosis of cardiovascular diseases. Postgrad Med 2008; 120: 34-41.
- Lopez IP, Marti A, Milagro FI, ZuletMdMde L, Moreno Aliaga MJ, Martinez A, De Miguel C. DNA microarray analysis of genes differentially expressed in diet-induced (cafeteria) obese rats. Obes Res 2003; 11: 188.
- Altunkaynak ME, Ozbek E, Altunkaynak BZ, Can I, Unal D. The effects of high-fat diet on the renal structure and morphometric parametric of kidneys in rats. J Anat 2008; 212: 845-852.
- Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. RegulPept 2008; 150: 26-32.
- Lomba A, Milagro FI, Garcia-Diaz DF, Campion J, Marzo F, Martinez JA. A high-sucrose isocaloric pair-fed model induces obesity and impairs NDUFB6 gene function in rat adipose tissue. J NutrigenetNutrigenomics 2009; 2:267-272.
- Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: Modelling the metabolic disorders of human obesity in rodents. Obesity 2007; 15: 798-808.
- Kanazawa M, Xue CY, Kageyama H, Suzuki E, Ito R. Effects of a high-sucrose diet on body weight, plasma triglycerides, and stress tolerance. Nutr Rev 2003; 61: S27-S33.
- Fukuchi S, Hamaguchi K, Seike M, Himeno K, Sakata T. Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats. ExpBiol Med 2004; 229: 486-493.
- Xu Y, Osborne BW, Stanton RC. Diabetes causes inhibition of glucose-6- phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol 2005; 289:1040-1047.