- Biomedical Research (2014) Volume 25, Issue 1
Prevention of Escherichia coli-induced diarrhea with microecological complex preparation in swine.
Xueqin Wan1, Zhixin Cui1, Hongming Li1, Jiping Zhang2,*, Zhongli Yu3, Yujing Geng31Department of Pathogenic Microbiology, Foshan University, Foshan, Guangdong, 528000, China
2Pharmaceutical Research Center, Foshan Hospital, Southern Medical University, Foshan, Guangdong, 528000, China
- *Corresponding Author:
- Jiping Zhang
Pharmaceutical Research Center
Foshan Hospital, Southern Medical University
No 78, Weiguo Road, Foshan City, Guangdong, 528000
China Email: fszjping@163.com
Accepted October 18 2013
Abstract
The aim of this work is to investigate the efficacy of non-antibiotic microecological complex preparation in the prevention of Escherichia coli - induced diarrhea in swine. A specific non-antibiotic microecological complex was prepared after sterilization and supplementation with probiotics. This preparation was used to prevent Escherichia coli (E.coli) - induced diarrhea in neonatal swine. Self-field epidemic strains were used to prepare vaccine and bacteriophages that were successfully applied to prepare the microecological complex preparation. Results showed no difference of cure rate of diarrhea between swine treated with microecological complex and antibiotic treatment (P>0.05). In the microecological complex treated swine, the recurrence rate of diarrhea was significantly lower than that in the antibiotic treated swine within 1 month (P<0.05). The microecological complex treatment could significantly reduce the incidence of diarrhea in pregnant swine (P<0.05), but treatment had no significant influence on the survival rate of neonatal swine (P>0.05). The microecological complex treatment could significantly reduce the incidence of diarrhea in weaned swine (P<0.05) but had no significant influence on the survival rate of neonatal swine (P>0.05). The specific microecological complex preparation is effective to treat the E. coli - induced diarrhea and can reduce the short-term recurrence of diarrhea in swine. The microecological complex treatment in pregnant swine can reduce the incidence of diarrhea in neonatal swine, and the complex used in neonatal swine can decrease the incidence of diarrhea in weaned swine.
Keywords
Pathogenic Escherichia coli, diarrhea in neonatal swine, virulent phage, non-antibiotic treatment
Introduction
Pathogenic Escherichia coli (E. coli) is a common and important pathogen causing zoonosis. They can infect humans via the animal source food or food contaminated by animal excrement, causing infectious food poisoning in humans and animals which is characterized by hemorrhagic enteritis. The pathogenic E. coli can be divided into enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), enterohaemorrhagic E. coli (EHEC) and enteroaggregative E. coli (EAEC) [1]. Since the EHEC induced food poisoning was identified in USA in 1982, the EHEC infection increases and spreads, and the outbreak and epidemic of EHEC induced diarrhea have been found in several countries including England, Canada and Japan. Especially, the EHEC induced diarrhea originating in Germany in May 2011 [2] spread more than in 16 countries including Europe and North America, and resulted in 4000 diarrhea cases and 50 related deaths. This caused worldwide panic and has been a public health emergency well within the memory of humans.
The major source of EHEC infection is animals, and the manifestations of EHEC infection in animals range from symptomless as a carrier to fetal diarrhea causing death. In China, pork is a major source of meat. E. coli- induced diarrhea is a common disease threatening the growth and development of neonatal swine. E. coli -induced diseases include yellow and white scour, edema disease and sepsis, and have a high morbidity and mortality [3]. In China, the major strategy for the prevention and treatment of E. coli induced diseases is addition of a large amount of antibiotics to the feed. This has detrimental consequences e.g. it influences the normal intestinal flora causing flora imbalance, leaves excessive drug residues in animal products affecting the quality of export products and leads to the occurrence of animal-derived drug resistant bacteria which hampers the prevention and treatment of infectious diseases in animals and humans.
Currently, microecological complex preparation (MECP) has been accepted as a strategy in the treatment of E. coli induced diarrhea in humans and animals [4]. In the present study, specific MECP [5] was prepared targeting the prevalent E. coli in neonatal swine. The virulent phages were separated and used to prepare the inactivated vaccine which was supplemented with probiotics to prepare the non-antibiotic MECP. After sterilization, supplementation with probiotics and establishment of immunity, MECP was prepared aiming to explore a strategy for the prevention and treatment of E. coli induced diarrhea in neonatal swine. Our findings may provide theoretical evidence for the prevention and treatment of E. coli induced diarrhea in organic pig production.
Materials and Methods
Samples
The swine were from the swine farm of Heap Bio- Technology Group and Nanzhuang County, and the Ethics Committee approved this study. Diarrheal feces were collected from the neonatal swine from the swine farm of Nanzhuang County in Foshan City and Heap Bio- Technology Group.
Reagents
Media, chemical reagents, trace fermentation tube, E. coli O-antigen factor serum and filters were purchased from the Haozhi Trading Co., Ltd in Guangzhou City, and E. coli O157 diagnostic serum was kindly provided by the Department of Laboratory, Nanfang Hospital.
Probiotics and accessories
Streptococcus faecalis and lactobacillus acidophilus were provided by the Heap Bio-Technology Group, and CaCO3 of Pharmaceutical Grade was from Hongxuan calcium carbonate Co., Ltd in Dongguan City.
Preparation of specific MECP
Separation and purification of pathogenic E. coli
The feces were inoculated onto SS medium followed incubation at 37℃ for 18-24 h. The E. coli which were pink, gram-negative and ++-- in the IMViC test were selected for O serotype identification. Once the serotype of E.coli was confirmed, the E. coli were seeded onto Nutrient broth agar followed by incubation at 37℃ for 18-24 h. The bacteria were collected and stored at 4℃ for use. Separation and purification of virulent phages of pathogenic E coli
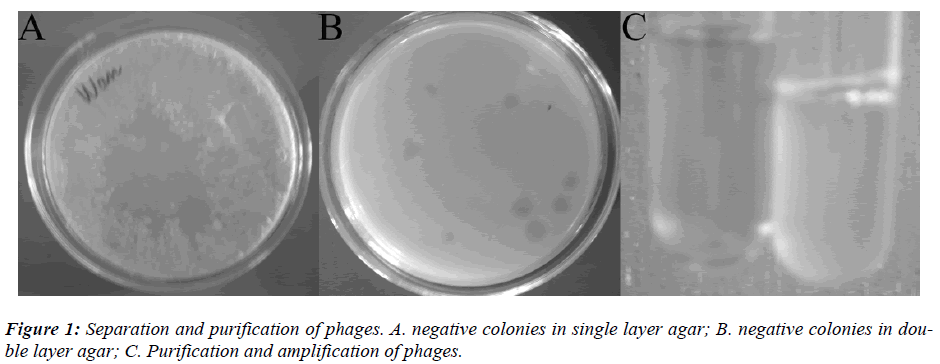
The above E coli were host bacteria. Single layer agar method was employed to separate the virulent phages of pathogenic E coli from the contaminated water. Then, double layer agar test was done to screen the virulent phages followed by purification and amplification. Then, the phage solution was prepared [6].
Preparation of inactivated vaccine with pathogenic E coli According to previously described (3), the inactivated vaccine was prepared. Adjuvant was not added because some components including probiotics in this preparation can serve as adjuvant.
Preparation of specific MECP
The inactivated bacteria solution was mixed with 30% alhydrogel brine solution followed by addition of 30% glycerol saline. After vortexing, the Streptococcus faecalis, Lactobacillus acidophilus and CaCO3 were added according to previously described. The MECP was added to a sterilized bottle and was stored at -20℃.
Treatment of diarrhea in neonatal swine
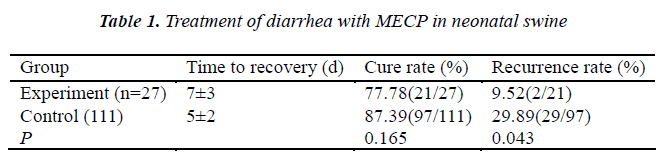
1) Selection and grouping of diarrhea neonatal swine: The neonatal swine and weaned swine (n=138) with simple diarrhea (no fever and organ failure) were recruited and numbered according to the time of diarrhea. The No 5 swine and animals with multiple of 5 (n=27) served as experiment group and the remaining 111 animals as controls. 2) Treatment: In the experiment group, the neonatal animals received intragastrical MECP at 50 ml/d/animal, and the weaned animals at 100 ml/d/animal. In the control group, animals were treated with antibiotics. Then, animals were observed until they recovered from diarrhea. The time to recovery, therapeutic efficacy and recurrence of diarrhea within 1 month were determined (Table 1).
Prevention of diarrhea in neonatal swine by treating pregnant swine
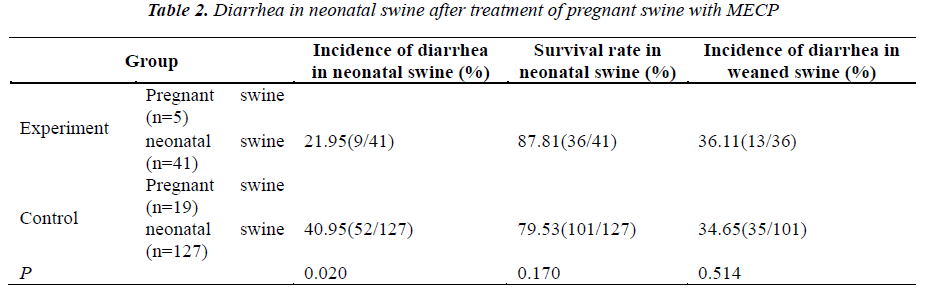
1) Grouping of pregnant swine: 28 pregnant swine were grouped: the NO5 animal and those with multiple of 5 served as experiment group and a total of 41 neonatal swine were born. The remaining 23 pregnant swine served as controls and a total of 127 neonatal swine were born by 19 mother swine. In addition, 29 neonatal swine born by 4 mother swine were treated and then observed. 2) Strategy for prevention: Pregnant swine were treated with MECP at 100 ml/d/animal from about 1 month before delivery to the end of breast-feeding period. In the control group, animals were normally fed. The incidence of diarrhea was detected in neonatal swine, and that was also determined within 1 month after weaning (Table 2).
Prevention of diarrhea in neonatal swine
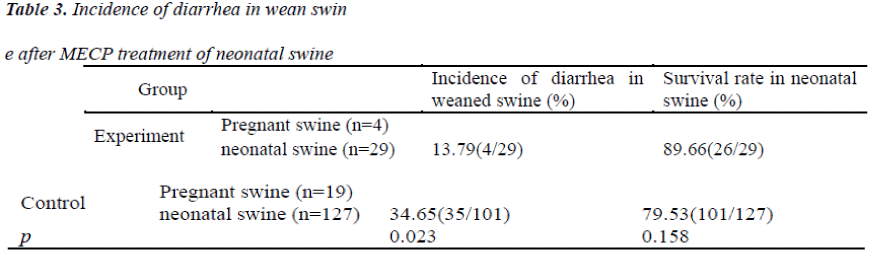
1) Grouping: 156 neonatal swine were grouped: NO5 animal and those with multiple of 5 served as experiment group (n=29) and the remaining 127 neonatal swine as controls. 2) Strategy for prevention: At 2 weeks before and after weaning, neonatal swine were treated with MECP at 100 ml/d/animal. Animals in the control group were normally fed. The incidence of diarrhea was determined after weaning (Table 3).
Statistical analysis
Data were expressed as percentage and compared with non-parametric Chi-Square Test. Statistical analysis was done with SPSS version 10.0. A value of P<0.05 was considered statistically significant.
Results
The feces were used to separate the pathogenic E coli. Serotyping showed the E coli was ETEC. The E coli O157 were not detectable. The pathogenic E coli were used as host bacteria for separation of virulent phages (Figure 1).
Prevention of diarrhea with MECP in swine
Determination
Discontinuation of diarrhea and recovery to normal diet
Discussion
The treatment of pathogenic E. coli - induced diarrhea has been a challenge to the veterinarians and medical doctors [7,8]. E coli is one of the normal flora in human intestine, and hence the treatment of pathogenic E coli may inevitably affect the normal flora in the intestine. In China, the Emergency Response Plan for Enterohemorrhagic E. coli (EHEC) Induced Diarrhea indicates that isolation and treatment are necessary for patients infected or suspiciously infected by EHEC. The major strategy is the supportive therapy in which MECP is used. In principle, antidiarrheal agents and drugs for peristalsis inhibition are not recommended. For patients infected or suspiciously infected by EHEC, antibiotic treatment is prohibited, and antibiotic is also not recommended in other diarrhea patients in the affected areas. For exposure subjects, prophylaxis is recommended, especially with MECP. In the present study, MECP containing coliphage, inactivated vaccine and intestinal probiotics were used to prevent and treat diarrhea in swine, which is crucial for the treatment of E. coli- induced diarrhea, and also provide evidence for the treatment and prevention of E. coli -induced diarrhea in humans.
Our results showed: 1) the self-field prevalent strains were used to prepare the vaccine and phages, which were then employed to prepare the MECP. This MECP when applied to treat diarrhea in swine, exerted an antibioticlike activity superior to only antibiotic treatment in preventing recurrence of diarrhea. 2) MECP treatment in pregnant swine reduced the incidence of diarrhea in neonatal swine. 3) MECP treatment in neonatal swine decreased the incidence of diarrhea in weaned swine. These findings suggest that MECP is effective to treat E. coli - induced diarrhea and can reduce the recurrence in short time; MECP treatment in pregnant swine may reduce the incidence of diarrhea in neonatal swine; MECP treatment in neonatal swine may decrease the incidence of diarrhea in weaned swine.
The antidiarrheal effect of specific MECP is related to its components: First, the prevalent E coli were used to separate the therapeutic phages, which have specific tracing and amplification of bactericidal effects and are not influenced by the drug resistance mutation in bacteria [9,10]. Bactericidal therapy with phages [11] is an ancient and effective antimicrobial therapy. The virulent phages have natural antimicrobial activity, and also possess the characteristics of individualized therapy. Especially, the antibiotic resistance increases. Thus, antimicrobial therapy with phages has been a focus in the development of antimicrobial agents. In the present study, phages were used to treat diarrhea as they reach the intestine and help to kill the pathogenic E coli. At the same time, the phages multiply significantly and may excrete via feces to kill the host bacteria in environment. In addition, the pathogenic E coli were used to prepare the inactivated vaccine, which has the activity of active immunization and may stimulate intestine to produce secretory antibodies against specific pathogenic E. coli. This increases the immunity of swine to defend a second infection with these pathogenic E. coli [12]. Finally, intestinal probiotics help to regulate the intestinal micro-ecological environment and elevate humoral immunity [4].
Taken together, the MECP is a non-antibiotic microecology and contains several antibacterial components, which promote their synergistic effect. Our findings demonstrate the effectiveness of MECP in the treatment of diarrhea in swine. MECP may reduce the application of antibiotics in animals, decrease the antimicrobial residues and increase the quality of animal products, which subsequently elevates the product competitiveness. In addition, the application of MECP also reduces the occurrence of animalderived drug resistant bacteria and attenuates the spread of bacteria between animals or between animals and humans. Moreover, MECP decrease the incidence of E. coli -induced diarrhea as a zoonosis. Our findings provide evidence for the development of complex biological antimicrobial preparations for treatment of diseases in humans.
Acknowledgments
The study was supported by Scientific Fund of Foshan University; General Program of the Spark Program of the Ministry of Science and Technology (2012GA780031); Science and Technology Program of Guangdong Province (0911152600004; 2010B031600150).
References
- Menne J, Nitschke M, Stingele R, Abu-Tair M, Beneke J, Bramstedt J, Bremer JP, Brunkhorst R, Busch V, Dengler R, Deuschl G, Fellermann K, Fickenscher H, Gerigk C, Goettsche A, Greeve J, Hafer C, Hagen- müller F, Haller H, Herget-Rosenthal S, Hertenstein B, Hofmann C, Lang M, Kielstein JT, Klostermeier UC, Knobloch J, Kuehbacher M, Kunzendorf U, Lehnert H, Manns MP, Menne TF, Meyer TN, Michael C, Münte T, Neumann-Grutzeck C, Nuernberger J, Pavenstaedt H, Ramazan L, Renders L, Repenthin J, Ries W, Rohr A, Rump LC, Samuelsson O, Sayk F, Schmidt BM,Schnatter S, Schöcklmann H, Schreiber S, von Sey- dewitz CU, Steinhoff J, Stracke S, Suerbaum S, van de Loo A, Vischedyk M, Weissenborn K, Wellhöner P, Wi- esner M, Zeissig S, Büning J, Schiffer M, Kuehbacher T; EHEC-HUS consortium. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case- control study. BMJ 2012; 345: e4565.
- Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Müller L, King LA, Rosner B, Buchholz U, Stark K, Krause G; HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011; 365: 1771- 80.
- Ren J, Yan X, Ai H, Zhang Z, Huang X, Ouyang J, Yang M, Yang H, Han P, Zeng W, Chen Y, Guo Y, Xiao S, Ding N, Huang L. Susceptibility towards enterotoxi- genic Escherichia coli F4ac diarrhea is governed by the MUC13 gene in pigs. PLoS One 2012; 7: e44573.
- Krause DO, Bhandari SK, House JD, Nyachoti CM. Response of Nursery Pigs to a Synbiotic Preparation ofStarch and an Anti-Escherichia coli K88 Probiotic. Appl Envir Microbiol 2010; 76: 8192-200.
- Wan XQ, Cui ZX, Li HM, et al. Preparation of com- pound microbial agents for specific prevention and treatment of E coli induced diarrhea in animals. PatentNo: CN102266552A; Preservation No: CCTCC M 2011068
- Wan XQ, Li HM, Cui ZX, et al. Way of administration to protect phage activity and its application. Patent No: CN102370669A
- Liu P, Piao XS, Thacker PA, Zeng ZK, Li PF, Wang D, Kim SW. Chito-oligosaccharide reduces diarrhea inci- dence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J Anim Sci2010; 88: 3871-9.
- Rivero MA, Passucci JA, Rodriguez EM, Parma AE. Role and clinical course of verotoxigenic Escherichia coli infections in childhood acute diarrhoea in Argen- tina. J Med Microbiol 2010; 59: 345-52.
- Jamalludeen N, Johnson RP, Shewen PE, Gyles CL. Evaluation of bacteriophages for prevention and treat- ment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet Microbiol 2009; 136: 135-41.
- Li HM, Li WK, Wan XQ, et al. Effects of E. coil resis-tant mutation on phage lysis. 2011 IEEE International Conference on Human Health and Biomedical Engi- neering: 1296-99.
- Haq IU, Chaudhry WN, Akhtar MN, Andleeb S, Qadri Bacteriophages and their Implications on Future Bio- technology: A Review. Virol J 2012; 10: 9.
- Santos MF, New RR, Andrade GR, Ozaki CY, Sant'Anna OA, Mendonça-Previato L, Trabulsi LR, Domingos MO. Lipopolysaccharide as an Antigen Tar- get for the Formulation of a Universal Vaccine against Escherichia coli O111 Strains. Clin Vaccine Immunol2010; 17: 1772-80.