- Biomedical Research (2011) Volume 22, Issue 4
Prevalence of inducible Clindamycin resistance among community-and hospital-associated Staphylococcus aureus isolates in a tertiary care hospi-tal in India
Jadhav Savita Vivek, Gandham Nageswari Rajesh, Sharma Mukesh, Kaur Manpreet, Misra R.N. , Matnani G.B. , Ujagare M.T., B. Saikat, Kumar Ajay
Department of Microbiology; Pad.Dr.D.Y.Patil Medical College and Research Centre, Pimpri, Pune, India.
Accepted date: April 28 2011
Abstract
Clinical failure of clindamycin therapy has been reported due to multiple mechanisms that include resistance to macrolide, lincosamide and strepto-gramin antibiotics. In vitro routine tests for clindamycin susceptibility may fail to detect inducible clindamycin resistance thus necessitating the need to detect such resistance by a simple D test on routine basis. Among 446 clinical isolates of Staphylococci studied, 145(32.5%) were MRSA and 301(67.84%) were MSSA. Of the 446 staphylococcal isolates 87 (19.50%) were resistant to erythromycin of which 41 (47.12%) showed inducible clindamycin resistance and belonged to the MLSBi phenotype. Among the 41 MLSBi phenotype 36(87.80%) were MRSA and 5(12.19%) were MSSA. Of the 36 MRSA 9(25%) were CA-MRSA and 27 (75%) were HA-MRSA. We conclude therefore, that D-test should be used as a mandatory method in routine disc diffusion testing to detect inducible Clinda-mycin resistance.
Key Words
Staphylococcus, inducible clindamycin resistance, D-test
Introduction
Staphylococcus aureus causes a variety of infections, ranging from skin and soft tissue infections to life threatening endocarditis [1]. Methicillin resistant Staphylococcus aureus (MRSA) is a major cause of nosocomial and community acquired infections [2]. Increasing frequency of Methicillin Resistant Staphylococcus aureus (MRSA) infections and changing patterns in antimicrobial resistance have led to renewed interest in the use of macrolide lincosamide – streptogramin B (MLSB) antibiotics to treat such infections. However, their widespread use has led to an increase in the number of Staphylococcus strains resistant to MLSB antibiotics [3]. The MLS family of antibiotics has three different mechanisms of resistance–target site modification, enzymatic antibiotic inactivation and macrolide efflux pumps. Clindamycin, a lincosamide antibiotic, is among the limited choice of antimicrobials effective against MRSA. There is concern about use of this antibiotic in the presence of Erythromycin resistance because of the possibility of induction of cross resistance among members of the macrolide, lincosamide, streptogramin B (MLSB) group [4]. As MRSA infections have become increasingly common in the community setting ,the development of empirical antimicrobial therapeutic strategies for Staphylococcal infections has become more problematic .Clindamycin has long been an option for treating both MSSA and MRSA infections.
However, expression of inducible resistance to clindamycin could limit the effectiveness of this drug. Demonstration of inducible MLSB phenotype in isolates that are susceptible to Clindamycin and resistant to Erythromycin is possible by using Double Disk diffusion agar inhibitory assay or D – test [5,6]. Data describing MLSB prevalence or clinical predictors of the presence of MLSBi among CA - MRSA and HA- MRSA isolates are quite limited in India. In the present study, we aimed to characterize MLSBi resistance in both hospital and community associated Staphylococcus aureus isolates, including MRSA and MSSA, at our institute.
Materials and Methods
The present study was conducted from Jan 2010 to Dec 2010.A total 446 isolates of Staphylococcus aureus isolated from various clinical specimens like pus, blood, wound swab, body fluids and urine were tested.
Staphylococcus aureus isolates were initially identified by the standard conventional methods [7,8,9 ] & followed by the screening for Oxacillin resistance using Oxacillin (1μg) on Muller Hinton agar supplemented with 2% NaCl followed by overnight incubation at 35°C [7].
Antimicrobial susceptibility testing were done by Kirby Bauer’s disc diffusion method on MullerHinton agar plates using Ciprofloxacin(5μg), Cefoxitin (30μg), Erythromycin (15μg) and Penicillin (10U) as per CLSI guidelines [8]. All antimicrobial susceptibility tests were interpreted in accordance with the 2007 guidelines published by CLSI. [MRSA isolates were designated as HA MRSA if the source patient had any of the following risk factors: a history of hospitalization, residence in a long term care facility (e.g. nursing home), dialysis, or surgery within one year to the date of specimen collection; growth of MRSA 48 h or more after admission to a hospital; presence of permanent indwelling catheter or percutaneous device at the time of culture; or prior positive MRSA culture report. If none of the above risk factors were present, the isolates were considered CA - MRSA.]
Phenotypic inducible resistance to clindamycin by D- test.
Isolates were plated on a Muller Hinton agar plate at a Mc Far land concentration of 0.5 to eventually cover the agar surface. Clindamycin and Erythromycin disks, containing 2 μg and 15μg each respectively were placed in the center of the plate separated by a distance of 15 cm between the edges. Plates were incubated at 37° C for 24 hr. Inducible resistance to Clindamycin was defined as blunting of the clear circular area of no growth around the Clindamycin disk on the side adjacent to the Erythromycin disk and was designated D - test positive. Absence of a blunted zone of inhibition was designated D - test negative. Three different phenotypes were interpreted as follows [8.9].
1. MS phenotype isolates showing circular zone of inhibition around Clindamycin (Zone size> 21mm) and resistance to Erythromycin (Zone size <13 mm) was labeled as MS phenotype.
2. Inducible MLSB phenotype Staphylococcal isolates showing resistance to Erythromycin (zone size <13 mm) and sensitive to Clindamycin (Zone size>21mm) giving D - shaped zone of inhibition around Clindamycin disc were labeled as Inducible MLSB phenotype.
3.Constitutive MLSB phenotype Staphylococcal isolates showed resistance to both Erythromycin (Zone size <13 mm) and Clindamycin (Zone size < 14mm) with circular shape of zone of inhibition if any around Clindamycin.
Results
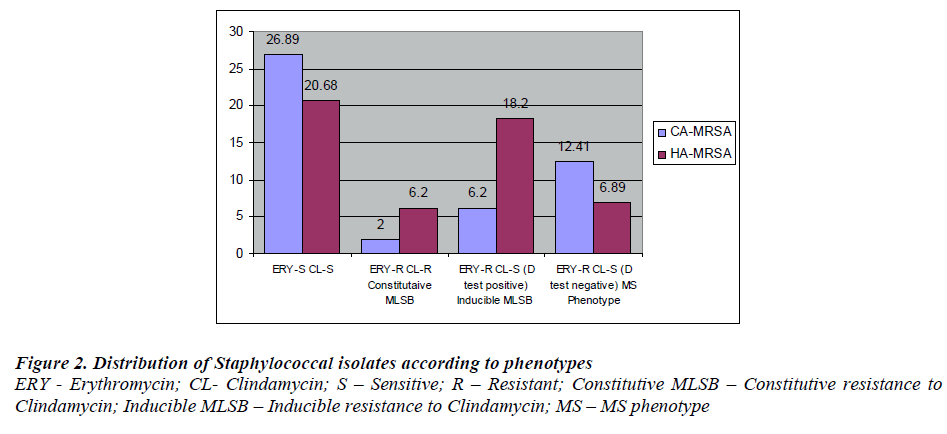
A total of 446 Staphylococcus aureus obtained from consecutive clinical specimens were included, consisting of 145 (32.51%) MRSA and 301 (67.48%) MSSA. Out of 446 Staphylococcus aureus 87 (19.50%) were resistant erythromycin. These isolates were subjected to D - test. 12 out of 87 showed resistance to both Erythromycin and Clindamycin indicating constitutive MLSB phenotype in MRSA.Constitutive MLSB phenotype was not detected in MSSA Of 87 , 41 (9.19%) showed positive D - test indicating inducible MLSB phenotype while 34 (7.62%) showed D test negative indicating MS phenotype. Among 41 MLSB phenotype, 36(87.80%) were from MRSA strains while 5 (12.19%) were MSSA strains. From a total 145 MRSA 36 (24.82%) were MLSB phenotypes [Fig. 4].
Of the 145 MRSA, 69(47.58%) were CA-MRSA & 76 (52.41%) were HA-MRSA. Among CA-MRSA, 9 (6.2%) were MLSB phenotype and from HA-MRSA 27 (18.52 %) were MLSB phenotype. Of 28 MS phenotype 18 (12.41 %) and 10 ( 6.89 % ) were from CA-MRSA & HA-MRSA respectively.[Fig. 3].
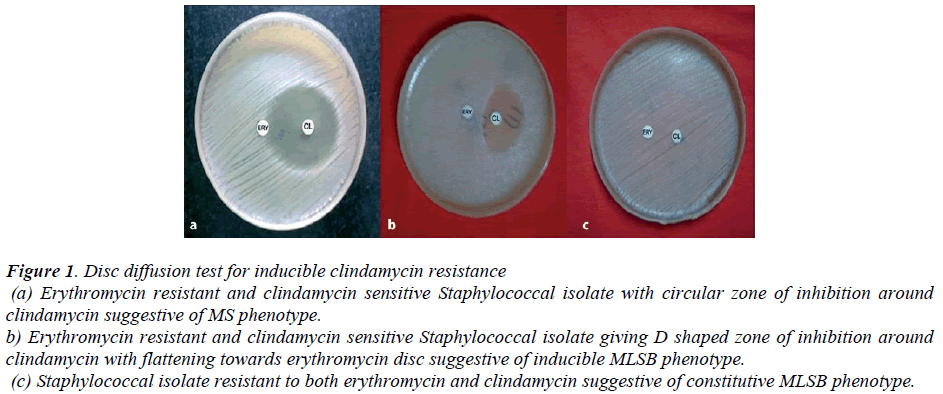
Figure 1: Disc diffusion test for inducible clindamycin resistance
(a) Erythromycin resistant and clindamycin sensitive Staphylococcal isolate with circular zone of inhibition around clindamycin suggestive of MS phenotype.
b) Erythromycin resistant and clindamycin sensitive Staphylococcal isolate giving D shaped zone of inhibition around clindamycin with flattening towards erythromycin disc suggestive of inducible MLSB phenotype.
(c) Staphylococcal isolate resistant to both erythromycin and clindamycin suggestive of constitutive MLSB phenotype.
Discussion
Clindamycin is an appealing option because of its proven efficacy, safety and convenience of parental and oral administration in patients. The possibility of inducible resistance is a concern. Clindamycin resistance can develop in Staphylococcal isolates with inducible phenotype and from such isolates, spontaneous constitutively resistant mutants have arisen both in vitro and in vivo testing during Clindamycin therapy.
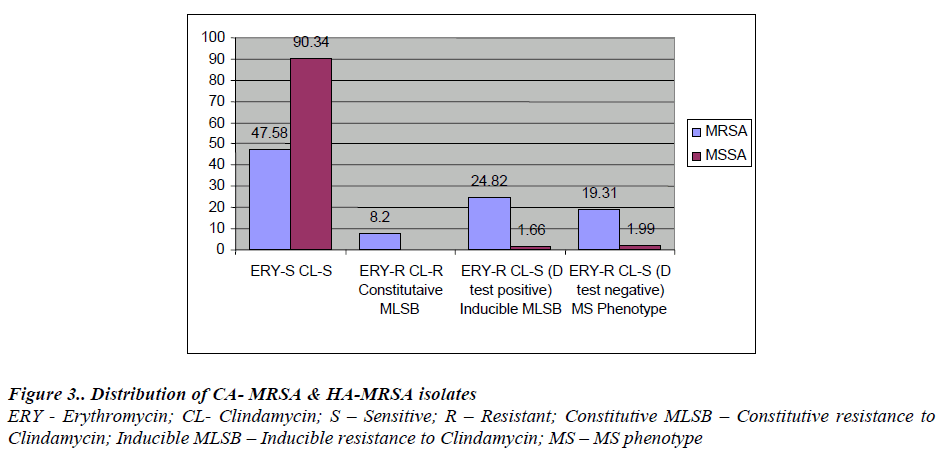
Our study suggests that there is a higher percentage of erythromycin resistant isolates 76 (52.41%) in MRSA as compared to MSSA 11 (3.6%). Out of 76 MRSA, 36 (24.82%) isolates were positive for ‘D’ test, 12 (8.2%) were constitutive MLSB and 28 (19.3%) were ‘D’ test negative. In 301 (67.48%) MSSA isolates, 5 (1.66%) were inducible Clindamycin resistant and 6 (1.99%) were ‘D’ test negative. This was in concordance with a few of the studies reported before by Yilmaz et al. [ 10] who found inducible resistance of 24.4% in MRSA and 14.8% in MSSA.
In a study conducted in Brazil [11,12] (Vander et al 2003) 11.3 % of Staphylococcus aureus were determined to have the inducible MLSB resistance phenotype, while Fiebelkorn et al,( 2003) [1] showed 29% to have inducible MLSB resistance phenotypes. In another study conducted in Turkey (Azap et al 2005), 5.7% among MRSA isolates, 3.6% MSSA isolates were determined to have inducible CL-R phenotype. In India Gadepalli et al. [5] showed it to be 30% in MRSA and 10% in MSSA, while Mohamed Rahabar et al [13] reported 22.6% in MRSA and 4% in MSSA, a trend that has been seen nationally.
Clinically, bacterial strains exhibiting MLSB have a high rate of spontaneous mutation to constitutive resistance, and the use of non inducer antibiotics such as clindamycin can lead to the selection of constitutive mutants and may result in clindamycin treatment failure [14, 15].
The emergence of resistance to multiple antibiotics among staphylococci has left very few therapeutic options for clinicians. A therapeutic decision is not possible with-out the relevant clinical and microbiological data. The increasing frequency of MRSA with in vitro inducible clindamycin resistance raises a concern of clindamycin treatment failures and this is where the D test becomes significant [16,17]
To conclude, the implementation of the D-test a simple, auxiliary method with routine antibiotic susceptibility testing, delineates inducible and constitutive clindamycin resistance. Consequently early detection helps in the use of clindamycin only in infections caused by truly clindamycin susceptible S. aureus and thus helps to avoid treatment failures.
References
- Fiebelkorn KR, Crawford SA, McElmeel ML, et al. Practical disc diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase negative Staphylococcus. J Clin Microbiol 2003; 41: 4740-4744.
- Frank AL, Marcinak JF, Mangat PD, et al. Community-acquired methicillin and Clindamycin-susceptible me-thicillin-resistant Staphylococcus aureus in children. Pediatric. Infect. Dis. J 1999; 18: 993-1000.
- Saiman L, O’Keefe M, Graham PI III, et al. Hospital transmission of communityacquired methicillinresistant Staphylococcus aureus among postpartum women. Clin. Infect. Dis.2003; 37: 1313-1319.
- Hussain FM, Boyle-Varva S, Bethel CD, et al. Currents trend in community-acquired methicillin-resistant Sta-phylococcus aureus at a tertiary care pediatric facility. Pediatr. Infect. Dis J 2000; 19: 1163-1166.
- Gadepalli R, Dhawan B, Mohanthy S, et al. Inducible clindamycin resistance in clinical isolates of Staphylo-coccus aureus. Indian J Med Res 2006; 123: 571-573.
- Steward CD, Raney PM, Morell AK, et al. Testing for induction of clindamycin resistance in erythromycin re-sistant isolates of Staphylococcus aureus. J Clin Micro-biol 2005; 43: 1716-17121.
- Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing: Sev-enteenth informational supplement. Vol 27. No. 1 Clinical Laboratory Standards Institute: 2007.
- Deotale V, Mediratta DK, Raut U,et al.Inducible clin-damycin resistance in Staphylococcus aureus isolated from clinical samples.Indain J Med Microbiology 2010; 28 (2): 124-126.
- Kloos WE, Banerman TL. Staphlococcus and Micro-coccus, Chapter22. In: Manual of clinical microbiol-ogy. 7th ed. Murray PR, Baron EJ, Pfaller MA, Tenoer FC, Yolken RH, editors. Washington DC. ASM Press; 1999: 264-282.
- Yiilmaz G, Aydin K, Iskender S,et al. Detection and prevalence of inducible clindamycin resistance in Staphylococci. J Med Microbiol 2007; 56: 342-345.
- Van Horn, K. G. & Toth, C.In Abstracts of the 103rd General Meeting of the American Society for Microbi-ology 2003, abstract C-074. Washington, DC:2003 American Society for Microbiology
- Rodrigues Prez LR, Caierao J, Souza Antunes AL,et al. Use of D test method to detect inducible clindamycin resistance in coagulase negative staphylococci (CoNS). Braz J Infect Dis 2007; 11: 186-188.
- Rahabar M, Hajia M. Inducible clindamycin resistance in Staphlococcus aureus: A cross sectional report. Pak J Biol Sci 2007; 10: 189-192.
- Ajantha GS, Kulkarni RD, Shetty J,et al. Phenotypic detection of inducible clindamycin resistance amongst Staphylococcus aureus isolates by using lower limit of recommended inter-disk distance. Indian J Pathol Mi-crobiol 2008; 51: 3768.
- Schreckenberger PC, Ilendo E, Ristow KL. Incidence of constitutive and inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci in a community and a tertiary care hospital. J Clin Microbiol 2004; 42: 2777-2779.
- Levin TP, Suh B, Axelrod P, et al. Potential clindamy-cin Resistance in clindamycin-susceptible, erythromy-cin-resistant Staphylococcus aureus: Report of a clini-cal failure. Antimicrob Agents Chemother 2005; 49: 1222-1224.
- Frank AL, Marcinak JF, Magnat PD, et al. Clindamy-cin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J 2002; 21, 530-534.